Professional Documents
Culture Documents
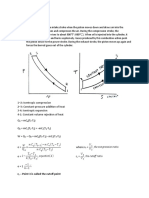
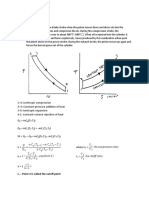
4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S Diagram
Uploaded by
Milan D SaintOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4.10 Brayton Cycle (Simple Gas Turbine Cycle) :: Fig.4.10. Brayton Cycle On P-V and T-S Diagram
Uploaded by
Milan D SaintCopyright:
Available Formats
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.10 Brayton Cycle (Simple Gas Turbine Cycle):
4' 1
Volume
(a)
3 4' 2 1
Entropy (b)
Fig.4.10. Brayton cycle on p-v and T-s diagram
Indian Institute of Technology Madras
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
The Brayton cycle is a theoretical cycle for simple gas turbine. This cycle consists of two isentropic and two constant pressure processes. Figure.4.10 shows the Brayton cycle on p-v and T-s coordinates. The cycle is similar to the Diesel cycle in compression and heat addition. The isentropic expansion of the Diesel cycle is further extended followed by constant pressure heat rejection. The thermal efficiency is given by,
th =
Heat added - Heat rejected Heat added
mCp ( T3 - T2 ) =1T4 - T1 T3 - T2
th =
mCp ( T3 - T2 ) - mCp ( T4 - T1 )
For isentropic processes, we have,
p T2 = 2 T1 p1
-1
p T3 = 3 and T4 p4
-1
But, p2 = p3 and p1 = p4, thus,
T T2 = 3 T1 T4
and we can write,
th = 1 T4 T =1- 1 T3 T2
T4 T v 1 = 1 = 2 = -1 T3 T2 v1 r
1 r - 1
v = 2 v1
-1
1 - 1 p 2
p1
1
= rp
( )
-1
th = 1 -
-1 rp
Indian Institute of Technology Madras
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Deviation of Actual Cycles from Air Standard Cycles:
The actual Otto and Diesel engines show marked deviations form the air-standard cycles described above. Above fig shows p-v-diagram for a high-speed diesel engine would be very similar in appearance. The main differences between the actual and theoretical cycles are as follows.
(a) Compression and expansion are not friction less adiabatic processes. A Certain amount of friction is always present and there is considerable heat transfer between the gases and cylinder wall. (b) Combustion does not occur either at constant volume or at constant pressure. (c) The thermodynamics properties of the gases after combustion are different than those of the fuel-air mixture before combustion.. (d) The combustion may be incomplete. (e) The specific heats of the working fluid are not constant but increases with temperature. (f) The cylinder pressure during exhaust process is higher than the atmosphere. As a result, more work has to be done by the piston on the gases to expel them out of the cylinder, than work done by the gases on the piston during the intake stroke. This difference in work, called pumping work, is represented by the pumping loop shown by hatched area. Note that this work is negative and represents loss of work called pumping loss.
Indian Institute of Technology Madras
You might also like
- 9 Atkinson CycleDocument3 pages9 Atkinson CyclecaptainhassNo ratings yet
- 7 Limited Pressure CycleDocument4 pages7 Limited Pressure CyclecaptainhassNo ratings yet
- 10 Lenoir CycleDocument2 pages10 Lenoir Cyclecaptainhass100% (1)
- B59TC Gas Power Plant NotesDocument23 pagesB59TC Gas Power Plant Noteshansdavid.aquino2004No ratings yet
- TDHT - 03 - Thermodynamic Cycles (Gas Steam) 1Document19 pagesTDHT - 03 - Thermodynamic Cycles (Gas Steam) 1djukwe keuzetienNo ratings yet
- Air Cycle RefrigerationDocument10 pagesAir Cycle RefrigerationashisNo ratings yet
- Otto Cycle, Fuels, Combustion PDFDocument39 pagesOtto Cycle, Fuels, Combustion PDFAchmad Rizal FirmansyahNo ratings yet
- Gas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasDocument39 pagesGas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasJoseph MwansaNo ratings yet
- Ideal CyclesDocument2 pagesIdeal CyclesSofia OrjuelaNo ratings yet
- Unit-Ii Diesel, Gas Turbine and Combined Cycle Power PlantsDocument71 pagesUnit-Ii Diesel, Gas Turbine and Combined Cycle Power Plantsrsankarganesh MECH-HICETNo ratings yet
- Cycle CalculationsDocument6 pagesCycle CalculationsmudassarhussainNo ratings yet
- Gas TurbineDocument7 pagesGas TurbineVishal BediNo ratings yet
- 11 - Dr. M. Atia - Gas Power Cycles-Dual CycleDocument20 pages11 - Dr. M. Atia - Gas Power Cycles-Dual CycleBahaa RaghebNo ratings yet
- crs2 1Document2 pagescrs2 1Chayut NuntadusitNo ratings yet
- Applied Thermodynamics and Heat Transfer - Unit IDocument37 pagesApplied Thermodynamics and Heat Transfer - Unit IArun ShankarNo ratings yet
- A Cooling Unit To Increase The Efficiency of Gas Turbine PlantDocument5 pagesA Cooling Unit To Increase The Efficiency of Gas Turbine PlantseventhsensegroupNo ratings yet
- Thermodynamics ConsidertionsDocument20 pagesThermodynamics ConsidertionsCoordinador LaboratoriosNo ratings yet
- In This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesDocument22 pagesIn This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesPardeep bainsNo ratings yet
- Basics of Gas Turbine Propulsion: Unit-1Document113 pagesBasics of Gas Turbine Propulsion: Unit-1NotesNo ratings yet
- JEE Advanced Heat and Thermodynamics Important QuestionsDocument26 pagesJEE Advanced Heat and Thermodynamics Important QuestionsMayank VermaNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument5 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Energy Systems: Gas Turbine System IIDocument23 pagesEnergy Systems: Gas Turbine System IIAbhiNo ratings yet
- Mechanical Upto Ic Engine SpecifocationnDocument22 pagesMechanical Upto Ic Engine SpecifocationnlechuznimaNo ratings yet
- CHE3161 Semester1 2010 Solutions PDFDocument14 pagesCHE3161 Semester1 2010 Solutions PDFkumiristineNo ratings yet
- Ideal Engine CycleDocument20 pagesIdeal Engine CycleMulugeta WoldeNo ratings yet
- Kmme Te101-3Document6 pagesKmme Te101-3ayla sözenNo ratings yet
- Lecture Notes OnDocument200 pagesLecture Notes Onananth k r100% (3)
- Compres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GDocument7 pagesCompres Turbine Heating (Combustion) 2: g-1 G g-1 G g-1 GMrityunjay KrNo ratings yet
- Applied Thermodynamics ME250: Submitted ToDocument12 pagesApplied Thermodynamics ME250: Submitted Tomad eye m00dyNo ratings yet
- City and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicsDocument20 pagesCity and Guilds 9210 Level 6 Module - Unit 128 Applied ThermodynamicsAlaa ShammaaNo ratings yet
- Gas Power CyclesDocument140 pagesGas Power CyclesMohammed AlsirajNo ratings yet
- 04 - Thermodynamic - Cycles - (Joule - B) PDFDocument8 pages04 - Thermodynamic - Cycles - (Joule - B) PDFAntonio Di FioreNo ratings yet
- Thermo Cycles PDFDocument29 pagesThermo Cycles PDFWaseem Abbas AttariNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Otto Cycle: Heat AddedDocument7 pagesOtto Cycle: Heat AddedMACLINo ratings yet
- CLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFDocument28 pagesCLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFVISHVESH GOYAL100% (1)
- Marine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayaDocument38 pagesMarine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayafaridNo ratings yet
- Chap 23Document15 pagesChap 23민규No ratings yet
- Power Cycles - Simple and RegenrationDocument24 pagesPower Cycles - Simple and Regenrationkumarmohit0203No ratings yet
- Assignment 2Document19 pagesAssignment 2Imalah UgoachanumNo ratings yet
- Calculation of Time Required To Fill Tanks With Compressed GasDocument4 pagesCalculation of Time Required To Fill Tanks With Compressed Gas1940LaSalleNo ratings yet
- AERO213: Aeroengines: AERO213 School of Engineering DR David JC Dennis 44831Document9 pagesAERO213: Aeroengines: AERO213 School of Engineering DR David JC Dennis 44831Ahmed ElgamalNo ratings yet
- Tegar Ari Widianto 16306141051 Penyelesaian SoalDocument10 pagesTegar Ari Widianto 16306141051 Penyelesaian SoalTegar Ari WidiantoNo ratings yet
- Gasturbine 1 160120155226Document52 pagesGasturbine 1 160120155226Muhammad Afif NaufalNo ratings yet
- Formula Sheet For Exam - April 2022Document5 pagesFormula Sheet For Exam - April 2022philip.groenemeijerNo ratings yet
- Principle of TurbomachineryDocument159 pagesPrinciple of TurbomachineryWalid MohammedNo ratings yet
- Gearbox TurbofanDocument7 pagesGearbox TurbofanRamanujam SankarNo ratings yet
- In This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesDocument23 pagesIn This Tutorial You Will Do The Following. Revise Gas Expansions in TurbinesHayderr HassNo ratings yet
- Basics of Gas TurbinesDocument5 pagesBasics of Gas TurbinesRammireszNo ratings yet
- Air Cycle Refrigeration:-Bell - Coleman CycleDocument21 pagesAir Cycle Refrigeration:-Bell - Coleman CycleSuraj Kumar100% (1)
- Non-Ideal Gas Turbines: 1 Adjustments For The Real WorldDocument4 pagesNon-Ideal Gas Turbines: 1 Adjustments For The Real WorldÁrpád PusztaszeriNo ratings yet
- L8 - AE242 01 Feb 24Document17 pagesL8 - AE242 01 Feb 24Shubhajit BiswasNo ratings yet
- SESM2021: Introduction To Energy Technologies Coursework I: Student Name: Eleni Tsigkogianni 24467669Document8 pagesSESM2021: Introduction To Energy Technologies Coursework I: Student Name: Eleni Tsigkogianni 24467669inele1No ratings yet
- Me423 Chapter 2Document76 pagesMe423 Chapter 2ddhiruNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Tables of Generalized Airy Functions for the Asymptotic Solution of the Differential Equation: Mathematical Tables SeriesFrom EverandTables of Generalized Airy Functions for the Asymptotic Solution of the Differential Equation: Mathematical Tables SeriesNo ratings yet
- Flange ALignmentDocument5 pagesFlange ALignmentAnonymous O0lyGOShYG100% (1)
- Sample Resume FinalDocument2 pagesSample Resume FinalSyed Asad HussainNo ratings yet
- Sharp AR-C172M ServiceM EN PDFDocument308 pagesSharp AR-C172M ServiceM EN PDFpiaggio_nrgNo ratings yet
- MSW - 1 - 2016 Munisicpal Solid Waste Rules-2016 - Vol IDocument96 pagesMSW - 1 - 2016 Munisicpal Solid Waste Rules-2016 - Vol Inimm1962No ratings yet
- Reinforcement Project Examples: Monopole - Self Supporter - Guyed TowerDocument76 pagesReinforcement Project Examples: Monopole - Self Supporter - Guyed TowerBoris KovačevićNo ratings yet
- 12-24VDC Powered Ignition System: N N N N N N NDocument2 pages12-24VDC Powered Ignition System: N N N N N N NLeinner RamirezNo ratings yet
- Dissertation Sample CommerceDocument4 pagesDissertation Sample CommerceBuyPapersOnlineForCollegeCanada100% (1)
- Life Buds: Audiophile Wireless Earbuds With Immersive Single Point Source AudioDocument2 pagesLife Buds: Audiophile Wireless Earbuds With Immersive Single Point Source AudioCecy LucasNo ratings yet
- 164 Dashboard Annotated Ver 2a W-InsertDocument1 page164 Dashboard Annotated Ver 2a W-Insertoleg164No ratings yet
- Needs Assessment Form Company Name: HRMO Address: Sta. Barbara Agoo, La UnionDocument2 pagesNeeds Assessment Form Company Name: HRMO Address: Sta. Barbara Agoo, La UnionAlvin LaroyaNo ratings yet
- 93 .SG, CBR, MDD, PI, SP - GRDocument11 pages93 .SG, CBR, MDD, PI, SP - GRChandra Prakash KarkiNo ratings yet
- Research On Restaurant DesignDocument20 pagesResearch On Restaurant DesignCrizalene Caballero100% (1)
- Psar Techspec Autologicsoftwaretechspecfor Psarvehicles en PF v2.0Document183 pagesPsar Techspec Autologicsoftwaretechspecfor Psarvehicles en PF v2.0PhatNo ratings yet
- Silverio Nuanez Verified ComplaintDocument10 pagesSilverio Nuanez Verified ComplaintMichael_Lee_RobertsNo ratings yet
- 1.doosan D1146Document1 page1.doosan D1146Md. ShohelNo ratings yet
- Design Calculation Sheet: Project No: Date: Sheet No.:1 1 Computed By: SubjectDocument1 pageDesign Calculation Sheet: Project No: Date: Sheet No.:1 1 Computed By: SubjectAbdelfatah NewishyNo ratings yet
- Design Calculation of Braking System (Landcruiser) : AbstractDocument4 pagesDesign Calculation of Braking System (Landcruiser) : AbstractDr. Aung Ko LattNo ratings yet
- 03 Pezeshki-Ivari2018 Article ApplicationsOfBIMABriefReviewADocument40 pages03 Pezeshki-Ivari2018 Article ApplicationsOfBIMABriefReviewAdanes1800No ratings yet
- Package Contents: Ariadni DivaDocument4 pagesPackage Contents: Ariadni DivaShadi AbdelsalamNo ratings yet
- Project DescriptionDocument5 pagesProject DescriptionM ShahidNo ratings yet
- Outsourced New Product DevelopmentDocument5 pagesOutsourced New Product Developmentvinnakota5No ratings yet
- General Milling Corp Vs CA (DIGEST)Document2 pagesGeneral Milling Corp Vs CA (DIGEST)Raima Marjian Sucor100% (1)
- Sick - Photoelectric 4-180 MM v2Document7 pagesSick - Photoelectric 4-180 MM v2Muhammad SumeetNo ratings yet
- Annexure 3 Courtesy Car AgreementDocument3 pagesAnnexure 3 Courtesy Car AgreementManishNo ratings yet
- Kathrein 739624Document2 pagesKathrein 739624anna.bNo ratings yet
- 1a. Create Your Yosemite Zone USB DriveDocument9 pages1a. Create Your Yosemite Zone USB DriveSimon MeierNo ratings yet
- KACE SeDocument63 pagesKACE SeAbdul RahimNo ratings yet
- HSE - Made Gde PanduDocument3 pagesHSE - Made Gde Pandurezki_WSNo ratings yet
- GR No. 188213 - January 11, 2016 FACTS: Herein Petitioner, Natividad Cruz, Was The Punong Barangay or Chairperson of BarangayDocument6 pagesGR No. 188213 - January 11, 2016 FACTS: Herein Petitioner, Natividad Cruz, Was The Punong Barangay or Chairperson of BarangayAilyn GaluraNo ratings yet