Professional Documents
Culture Documents
1st Law of Thermodynamic Explained
Uploaded by
Yuichi SahachaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1st Law of Thermodynamic Explained
Uploaded by
Yuichi SahachaCopyright:
Available Formats
1st Law of Thermodynamic
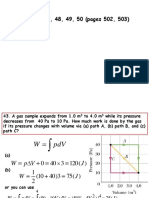
43 In Fig. 18-36, a gas sample expands from V0 to 4.0V0 while its pressure decreases from p0 to p0/4.0. If V0 = 1.0 m3 and p0 = 40 Pa, how much work is done by the gas if its pressure changes with volume via (a) path A, (b) path B, and (c) path C?
Figure 18-36 Problem 43.
47
When a system is taken from state i to state f along path iaf in Fig. 1839, Q = 50 cal and W = 20 cal. Along path ibf, Q = 36 cal. (a) What is W along path ibf? (b) If W = -13 cal for the return path fi, what is Q for this path? (c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, what is Q for (d) path ib and (e) path bf?
Figure 18-39 Problem 47.
49
Figure 18-41 represents a closed cycle for a gas (the figure is not drawn to scale). The change in the internal energy of the gas as it moves from a to c along the path abc is -200 J. As it moves from c to d, 180 J must be transferred to it as heat. An additional transfer of 80 J to it as heat is needed as it moves from d to a. How much work is done on the gas as it moves from c to d?
Figure 18-41 Problem 49.
50 A lab sample of gas is taken through cycle abca shown in the p-V diagram of Fig. 18-42. The net work done is +1.2 J. Along path ab, the change in the internal energy is +3.0 J and the magnitude of the work done is 5.0 J. Along path ca, the energy transferred to the gas as heat is +2.5 J. How much energy is transferred as heat along (a) path ab and (b) path bc?
Figure 18-42 Problem 50.
Suppose that on a linear temperature scale X, water boils at -53.5X and freezes at 170X. What is a temperature of 340 K on the X scale? (Approximate water's boiling point as 373 K.)
You might also like
- PrimeraLey PVDocument5 pagesPrimeraLey PVISAI dali Perez navaNo ratings yet
- Exercice Thermodynamics PART IDocument8 pagesExercice Thermodynamics PART Ichitl.23bi14075No ratings yet
- Physics I Problems PDFDocument1 pagePhysics I Problems PDFbosschellen0% (1)
- ExtraProbPhys2Ch3 HeatWorkLaw1Document1 pageExtraProbPhys2Ch3 HeatWorkLaw1Duy AnhNo ratings yet
- ExercisesDocument19 pagesExercisesNhật MinhNo ratings yet
- Physics Chapter 19 Class ProblemsDocument3 pagesPhysics Chapter 19 Class ProblemsRuba AlNo ratings yet
- Test-28 Thermo, KTG WADocument4 pagesTest-28 Thermo, KTG WAumang dhandhaniaNo ratings yet
- ANSWER: (A) 1.2 X 10 J (B) 75 J (C) 30 JDocument2 pagesANSWER: (A) 1.2 X 10 J (B) 75 J (C) 30 JHiếu VũNo ratings yet
- Thermodynamics Level 1: Temperature Scale and Work Done in Different ProcessesDocument7 pagesThermodynamics Level 1: Temperature Scale and Work Done in Different ProcessesViren Patel50% (2)
- Important Thermodynamics QuestionsDocument6 pagesImportant Thermodynamics QuestionsAditya SallyNo ratings yet
- Gas undergoes cyclic processDocument2 pagesGas undergoes cyclic processAshish GuptaNo ratings yet
- Thermodynamics and Kinetic Theory of Gases ConceptsDocument4 pagesThermodynamics and Kinetic Theory of Gases ConceptsTarun GuptaNo ratings yet
- AP Problems Database UhrichDocument18 pagesAP Problems Database UhrichMagesh KumarNo ratings yet
- Lecture05 P2Document24 pagesLecture05 P2Phạm Thiên LongNo ratings yet
- Problems Chapter 1 Sec BDocument7 pagesProblems Chapter 1 Sec BSana AshfaqNo ratings yet
- Basic ThermodynamicsDocument8 pagesBasic ThermodynamicsVivek VermaNo ratings yet
- Thermodynamics HomeworkDocument3 pagesThermodynamics HomeworkMinhNo ratings yet
- Thermodynamics Worked Examples PDFDocument20 pagesThermodynamics Worked Examples PDFJoshua Edokpayi100% (1)
- UniversityPhysicsVolume2 Ch03Document11 pagesUniversityPhysicsVolume2 Ch03Dominador RomuloNo ratings yet
- 9a-Thermodynamics MC Practice ProblemsDocument14 pages9a-Thermodynamics MC Practice ProblemsFrancis Nicole V. QuirozNo ratings yet
- Energy BalanceDocument28 pagesEnergy BalanceEian HawNo ratings yet
- Test Your C SkillsDocument8 pagesTest Your C SkillsBharadwaj SubramaniamNo ratings yet
- Microsoft Word Chapter 15Document20 pagesMicrosoft Word Chapter 15Shashank ShekharNo ratings yet
- KTG & Thermodynamics (QB) For-FDocument8 pagesKTG & Thermodynamics (QB) For-FRaju SinghNo ratings yet
- Phy 1Document43 pagesPhy 1Garlapati Srinivasa RaoNo ratings yet
- SOAL Modul 9 Fidas IA - 2022 2023 enDocument2 pagesSOAL Modul 9 Fidas IA - 2022 2023 enMario Hermawan SetiadiNo ratings yet
- Instructions: 1. Use Excel 2. Express Your Final Answer in 2 Decimal PlacesDocument6 pagesInstructions: 1. Use Excel 2. Express Your Final Answer in 2 Decimal PlaceslukeNo ratings yet
- DPP-18 (Thermodynamics)Document4 pagesDPP-18 (Thermodynamics)Dushyanth S JNo ratings yet
- DPP Thermodynamics Nitesh DevnaniDocument26 pagesDPP Thermodynamics Nitesh DevnaniaadilNo ratings yet
- TERM 2 Important Questions of Class 11thDocument9 pagesTERM 2 Important Questions of Class 11thAdityaNo ratings yet
- Refrigerant 134a vapor pressure drop heat transfer processDocument4 pagesRefrigerant 134a vapor pressure drop heat transfer processCarla CoronaNo ratings yet
- DuihfuifhuifhuiDocument2 pagesDuihfuifhuifhui張耀恩No ratings yet
- Question From MoranDocument12 pagesQuestion From MoranandrewjovellanaNo ratings yet
- Example 4Document41 pagesExample 4Akatew Haile MebrahtuNo ratings yet
- Solution Part 1 (2023)Document6 pagesSolution Part 1 (2023)01khanh26No ratings yet
- Thermodynamics Homework on Gas Processes and CyclesDocument20 pagesThermodynamics Homework on Gas Processes and CyclesBrandy TranNo ratings yet
- HeatDocument12 pagesHeatMuneer KaleriNo ratings yet
- ADHWAT WORLD ACADEMY PHYSICS TESTDocument5 pagesADHWAT WORLD ACADEMY PHYSICS TESTUtkarsh VaishNo ratings yet
- Thermo HW SolutionsDocument35 pagesThermo HW SolutionsekantikdevoteeNo ratings yet
- MT1 Thermodynamics Practice QuestionsDocument3 pagesMT1 Thermodynamics Practice Questionsmehmet candanNo ratings yet
- MidtermDocument3 pagesMidtermMohamed AliNo ratings yet
- Physics Thermodynamics ChapterDocument39 pagesPhysics Thermodynamics ChapterMuhammad SolehinNo ratings yet
- Tutorial Four-TutorialDocument4 pagesTutorial Four-Tutorialhagt813No ratings yet
- Thermodynamics DPP 8Document4 pagesThermodynamics DPP 8shubhamauddhyaNo ratings yet
- PHYF144 Tutorial Questions UpdatedDocument16 pagesPHYF144 Tutorial Questions UpdatedAnonymous KUuLddnO98No ratings yet
- Exercise-01 Check Your GraspDocument22 pagesExercise-01 Check Your GraspDeborshi ChakrabartiNo ratings yet
- AITS PT II MainDocument21 pagesAITS PT II MainSurya Prakash100% (1)
- Refrigeration Cycle Performance CalculationsDocument22 pagesRefrigeration Cycle Performance CalculationsSaman Brookhim100% (1)
- Physical Chemistry Assignment - Faculty of Chemical & Natural Resources EngineeringDocument2 pagesPhysical Chemistry Assignment - Faculty of Chemical & Natural Resources EngineeringSiti HajarNo ratings yet
- Homework 1Document4 pagesHomework 1Itzel A. GuerreroNo ratings yet
- TB Chapter12Document14 pagesTB Chapter12smohanty20No ratings yet
- Boardwork - Closed SystemDocument12 pagesBoardwork - Closed SystemJannineNo ratings yet
- Question PART 4 (2023)Document7 pagesQuestion PART 4 (2023)Phong ĐặngNo ratings yet
- Physics I ProblemsDocument1 pagePhysics I ProblemsbosschellenNo ratings yet
- SSC Thermal EngineeringDocument47 pagesSSC Thermal EngineeringSteph Dela MujerNo ratings yet
- Thermodynamics Equations and ConceptsDocument48 pagesThermodynamics Equations and ConceptsMarvic Oquindo Unay43% (7)
- 11th Physics Ch-11 - Thermodynamics (SQP) 2023-24Document10 pages11th Physics Ch-11 - Thermodynamics (SQP) 2023-24Mahalaksshmi .DNo ratings yet
- XII Physics Irfan Sanjrani-1Document3 pagesXII Physics Irfan Sanjrani-1jaipal singhNo ratings yet
- Electrical and Electronic Principles 3 Checkbook: The Checkbook SeriesFrom EverandElectrical and Electronic Principles 3 Checkbook: The Checkbook SeriesNo ratings yet
- Landscape and Post ProcessingDocument58 pagesLandscape and Post ProcessingYuichi SahachaNo ratings yet
- How To Write A Spi ProgramDocument52 pagesHow To Write A Spi ProgramYuichi Sahacha100% (1)
- Chapter - 2 - Part2 - DT Signals & Systems PDFDocument12 pagesChapter - 2 - Part2 - DT Signals & Systems PDFYuichi SahachaNo ratings yet
- Flavonoid, Carotenoid, and Chlorophyll Pigments in MelonDocument118 pagesFlavonoid, Carotenoid, and Chlorophyll Pigments in MelonSeleneArthurNo ratings yet
- Eng ProjectDocument10 pagesEng ProjectYuichi SahachaNo ratings yet
- Binary-to-BCD Converter: Basic IdeaDocument5 pagesBinary-to-BCD Converter: Basic IdeaNguyễn LongNo ratings yet