Professional Documents
Culture Documents
C Coding Cheat Sheet
C Coding Cheat Sheet
Uploaded by
cjmallonCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C Coding Cheat Sheet
C Coding Cheat Sheet
Uploaded by
cjmallonCopyright:
Available Formats
General Chemistry Reference Sheet
This reference sheet addresses some of the more peculiar
pieces of information that need to be memorized in a gen-
eral chemistry course. It also contains a simple set of es-
sential formulas in chemistry with cautions, explanations,
and general tips.
This sheet is meant to be as concise as possible, and many
information in the textbook is left out in favor of cautions
and tips. This sheet is, therefore, best used as a supple-
ment to, not a replacement of, the textbook.
SI Fundamental Units
Mass Kilogram (kg)
Length Meter (m)
Time Second (s)
Temperature Kelvin (K)
Amount of substance Mole (mol)
Electric current Ampere (A)
Luminous intensity Candela (cd)
Atomic Experiments and Models
J. J. Thomson Discovered e
; Cathode ray
Plum pudding model
R. A. Millikan Measured charge of e
; Oil drop
H. Becquerel/M. Curie Discovered radioactivity
E. Rutherford Discovered , , and rays
Discovered nucleus; Gold foil experiment
J. Chadwick Discovered neutrons
N. Bohr Bohr model (electron orbits)
Quantum mechanicists Quantum model
Polyatomic Ions
NH4
+
ammonium OH
hydroxide
CN
cyanide C2O4
2
oxalate
O2
2
peroxide CNO
cyanate
HSO4
hydrogen sul-
fate
C2H3O2
acetate
SCN
thiocyanate NO3
nitrate
SO3
2
sulte ClO4
perchlorate
CO3
2
carbonate ClO3
chlorate
PO4
3
phosphate ClO
hypochlorite
S2O3
2
thiosulfate HPO4
2
hydrogen
phosphate
CrO4
2
chromate H3O
+
hydronium
Cr2O7
2
dichromate PO3
3
phosphite
MnO4
permanganate Hg2
2
mercury (I)
N3
azide C2
2
carbide
C4H4O6
2
tartrate S2
2
disulde
O2
superoxide AsO3
3
arsenite
PO2
3
hypophosphite AsO4
3
arsenate
SiO3
2
silicate P2O7
4
pyrophosphate
Ionic Solubility Chart
Soluble Exceptions
NO3
None
CH3COO
None
Cl
Ag
+
, Hg2
2+
, Pb
2+
Br
Ag
+
, Hg2
2+
, Pb
2+
I
Ag
+
, Hg2
2+
, Pb
2+
SO4
2
Sr
2+
, Ba
2+
, Hg2
2+
, Pb
2+
Insoluble Exceptions
S
2
NH4
+
, alkali metal cations, Ca
2+
,
Sr
2+
, Ba
2+
CO3
2
NH4
+
, alkali metal cations
PO4
3
NH4
+
, alkali metal cations
OH
NH4
+
, alkali metal cations, Ca
2+
,
Sr
2+
, Ba
2+
Strong Acids and Bases
Strong acids and bases dissociate in water completely.
Strong Acids Strong Bases
HCl HClO4 Alkali metal hydroxides
HBr HNO3 Ca(OH)2
HI H2SO4 Sr(OH)2
HClO3 Ba(OH)2
Activity Series
Metals below H
+
cannot react with acids to form H2. More
active metals are better reducing agents.
From most active to least active:
Li
+
, K
+
, Ba
2+
, Ca
2+
, Na
+
, Mg
2+
, Al
3+
, Mn
2+
, Zn
2+
,
Cr
3+
, Fe
2+
, Co
2+
, Ni
2+
, Sn
2+
, Pb
2+
, H
+
, Cu
2+
, Ag
+
,
Hg
2+
, Pt
2+
, Au
3+
Flame Colors
Calcium Brick red
Copper (I) Blue
Copper (II) Green or blue-green
Potassium Lilac
Lithium Dark red
Sodium Bright yellow
Strontium Red
Barium Light green
Iron (III) Gold
Cesium BlueViolet
Indium Blue
Lead Blue
Rubidium RedViolet
Phase Changes
From solid From liquid From gas
To solid - freezing deposition
To liquid melting - condensation
To gas sublimation vaporization -
Solution Colors
Copper (II) Blue
Nickel Green
Permanganate Purple
Chromate Yellow
Dichromate Orange
Iron (II) Light blue
Iron (III) Rusty yellow
Thermodynamic Laws
First Law: Energy cannot be created nor destroyed. It
can only be transferred in the form of either heat or work.
Second Law: Any spontaneous reaction increases the en-
tropy of the universe.
Third Law: An ideal solid crystal at 0 K has an entropy
of 0.
Thermodynamic Formulas
Standard thermodynamic conditions 298 K; 1 atm; 1 M
Kinetic energy K = mv
2
/2
Electrostatic potential energy UE = (kCQ1Q2)/d
Internal energy E = q +w
Enthalpy H = E +PV
Specic heat s = q/(m T)
Entropy in reversible reaction Ssystem = (H)/T
Ssurrounding = (H)/T
Microstate-entropy relationship S = k ln W
Gibbs free energy G = H TS
Gibbs free energy change G = H TS
G = G
+RT ln Q
Hesss Law Htotal = Hi
Constants
Boltzmanns constant kB = 1.381 10
23
m
2
kg s
2
K
1
Coulombs constant kC = 1/(40) = 8.988 10
9
J m/C
2
Avogadros number NA = 6.022 10
23
mol
1
Faradays constant F = 9.649 10
4
C/mol
Plancks constant h = 6.626 10
34
Js
Ideal gas constants R = 0.0821 (L atm)/(mol K)
R = 8.314 J/(mol K)
Vacuum permittivity 0 = 1/(0c
2
) = 8.854 10
12
F/m
Vacuum permeability 0 = 1.257 10
6
NA
2
Atomic mass 1 amu = 1.661 10
24
g
Electron charge e = 1.602 10
19
C
Electronvolt 1 eV = 1.602 10
19
J
Atmospheric pressure 1 atm = 1.013 10
5
Pa
Absolute zero 0 K = -273.15
C
Speed of light in vacuum c = 2.998 10
8
m/s
Quantum Mechanical Formulas
Energy of a quantum E = h
Wavelength-frequency relationship c =
Probability distribution PV =
V
|(x, y, z)|
2
dxdydz
Laws of Quantum Mechanics
Heisenbergs Uncer-
tainty Principle
x p h/2
Corollary: It is impossible to de-
termine both the position and
the momentum for a suciently
small particle like an e
.
Pauli Exclusion Prin-
ciple
No two e
in an atom can share
the same set of four quantum
numbers.
Corollary: A suborbital can hold
a maximum of 2e
.
Hunds Rule Energy is the lowest when the
number of e
with the same spin
is maximized.
Corollary: e
will rst half-ll
all the empty suborbitals, then
go back and ll the half subor-
bitals.
Quantum Numbers of e
Principal (n) The energy shell of the e
, e.g. 4 in
4d
1
.
Azimuthal (l) The suborbital shape, with s=0,
p=1, d=2, f=3, e.g. 2 in 4d
1
.
Magnetic (ml) The suborbital, ranging from l to
l, e.g. 2 in 4d
1
.
Spin (ms) The spin of e
. Two e
in the same
suborbital has either 1/2 or 1/2.
Molecular Geometry
Hybridization Nonbonding Geometry
electrons
sp 0 linear
sp
2
0 trigonal planar
1 bent
sp
3
0 tetrahedral
1 trigonal pyramidal
2 bent
sp
3
d 0 trigonal bipyramidal
1 seesaw
2 T-shaped
3 linear
sp
3
d
2
0 octahedral
1 square pyramidal
2 square planar
Atomic Properties
Atomic size
1
2
the distance between two ad-
jacent nucleii.
Ionic size Cations are smaller than their
parent atoms. Anions are
larger than their parent atoms.
N-th ionization energy The energy required to re-
move the n-th electron from a
ground state gaseous atom.
Electron anity The energy released by adding
an electron to a gaseous atom.
Metallic character The qualities of a metal.
Metals are shiny and heat and
electricity-conducting; they
have malleble solid form, form
basic ionic oxides, and tend
to form cations in an aqueous
solution.
Periodic Properties
Property Left to Right Top to Bottom
Atomic size Decreasing Increasing
Ionization energy Increasing Decreasing
Electron anity Large if adding
to a previously
empty orbital
No apparent
change
Metallic character Decreasing Increasing
Types of Crystalline Solids
Type IMFs Properties
Molecular Van der Waals forces,
dipole-dipole inter-
actions, hydrogen
bonds
Soft, low melting
point, poor conduc-
tion
Covalent-
network
Covalent bonds Very hard, high
melting point, poor
conduction
Ionic Electrostatic interac-
tions
Hard, high melting
point, poor conduc-
tion
Metallic Metallic bonds Soft to very hard,
low to very high
melting point, ex-
cellent conduction
Boiling Points of Molecular Compounds
The relative boiling points of molecular compounds can be
determined by their IMFs. The stronger the IMFs are,
the higher the boiling point. (Note that linear compounds
like straight-chain hydrocarbons have higher van der Waals
forces than non-linear compounds because their molecules
have a greater area of contact.)
IMFs in Molecular Solids
London dispersion
force (van der Waals
forces, induced dipole-
dipole interactions)
Interactions between dipoles
partially charged through the
movement of shared electron.
Presents in all compounds.
Weakest of the three.
Dipole-dipole interac-
tions
Interactions between dipoles
partially charged through the
electronegativity dierence of
two bonding atoms.
Hydrogen bonds A special kind of dipole-dipole
interactions present in com-
pounds that have hydrogen
and either oxygen or nitrogen.
Strongest of the three.
Acid-Base Theories
Arrhenius Brnsted-
Lowry
Lewis
Acids [H
+
] >[OH
] Proton
donors
Electron
acceptors
Bases [OH
] >[H
+
] Proton
acceptors
Electron
donors
Acids have H
+
Hydrogen
atom
Electron
accepting
atom
Bases have OH
Unshared
electron pair
Unshared
electron
pair
Acid + Base
Salt + H2O Conjugate
acid +
Conjugate
base
Kinetic Molecular Theory (KMT)
There is a very large number of particles;
Particles are in constant random motion and collide con-
stantly with the wall;
Collisions of particles with the wall are perfectly elastic;
Particles exert no force upon each other.
Properties of Solutions
Solvation The uniform dispersion of a solute in a solvent.
Hydration Solvation in water.
Crystallization The reverse reaction of solvation.
Saturated A solution in equilibrium.
Unsaturated A solution with less solute than saturation.
Supersaturated A solution with more solute than
saturation. (Will undergo crystallization
if a crystal seed is present.)
Miscible Two liquids that dissolve in any proportion.
Henrys Law S P (S: solubility)
2
Colligative Properties
Physical properties of a solution that depends on the con-
centration of solutes. More solutes will lead to:
1. Lower vapor pressure: PA = XAP
A
(Raoults
Law)
2. Higher boiling point: Tb = T
b
+kbm (Molality)
3. Lower freezing point: Tf = T
f
kf m (Molality)
4. Higher osmotic pressure: = RT M (Molarity)
Colligative properties also depend on the van t Ho factor
(i = number of particles after reaction / number of parti-
cles before reaction). The greater i is, the more colligative
properties it exerts on the solution.
Reaction Rate
The reaction rate r = d[X]/dt can be determined from the
reaction by the rate law
r = k[A]
a
[B]
b
...
Where a, b, etc. are reaction orders for the reactants. Re-
action orders can only be determined experimentally, be-
cause reactions will in theory go through several steps, the
slowest of which is the rate-determining step. Reaction or-
der is determined by the number of atoms participating in
the rate-determining step. The sum of these orders is the
overall order.
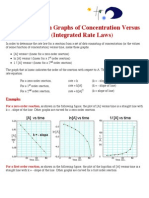
Concentration function [X] can be determined as
[X]t =
t
0
rd + [X]0
Therefore, for rst-order reactions,
ln[X]t = kt + ln[X]0
Graphically, t is proportional to ln[X]t with the slope k.
And for second-order reactions,
1
[X]t
= kt +
1
[X]0
Graphically, t is proportional to 1/[X]t with the slope k.
Reaction Half-time
The half-time of a reaction is the amount of time needed
to consume half of the reactants. It is denoted t
1/2
.
For rst order reactions, t
1/2
0.693/k. For second order
reactions, t
1/2
= 1/(k[X]0).
Activation Energy
Collision model Reactions occur as a result of collisions
between molecules.
Activation energy (Ea) The minimum energy required
for a reaction to occur.
Arrhenius equation ln k = ln AEa/RT
(This means that ln k 1/T)
Activation energy is lowered when a catalyst is present. In-
organic catalysts usually provide a site on which reactants
can adsorb; organic catalysts, or enzymes, bind specic to
substrate molecules (lock-and-key).
Spectrophotometry of Concentration
Beers law, A = lc, relates concentration and light absorp-
tion.
Absorbance (A) log
10
(I/I0) (liquids)
ln(I/I0) (gases)
Absorption coecient () Depends on the solution.
Length of path (l) The length of the path travelled
by light.
Concentration (c) The concentration of the solution.
In spectrophotometry, the length of path is xed. There-
fore, when using the same solution, A c.
Gas Laws
STP 273 K; 1 atm
Boyles Law P 1/V
Charless Law P T
Avogadros Law P n
Ideal Gas Equation PV = nRT
Law of Partial Pressure Pn = XnPt
Eusion Rate u =
(3RT)/M
Grahams Law u1/u2 =
M2/M1
Density Formula d = (PM)/(RT)
Deviation from Ideal Behavior (PV )/(RT)
Van der Waals Equation
P + (n
2
a)/V
2
(V nb) = nRT
Clausius-Clapeyron Equation ln P = Hvap/(RT) +C
Caution: When using the eusion rate formula, the R
value must be in joules (8.314), and the M value must be
converted to kg/mol.
Equilibrium Formulas
Ion-product constant of water
Kw = [H
+
][OH
] = 1.0 10
14
(278 K)
Henderson-Hasselbalch equation
pH = pKa + log([base]/[acid])
Van t Ho equation d(ln K)/dT = (H
)/(RT
2
)
ln K = H
/(RT) + S
/R
Concentration
Notation Denition
Molarity (M) (Moles solute)/(Liters solution)
Molality (m) (Moles solute)/(Kilogram solvent)
Mole fraction (X) (Moles solute)/(Moles solution)
Mass percentage (Mass of solute)/(Mass of solution)
Volume percentage (Volume of solute)/(Volume of solu-
tion)
Caution: It is an extremely common mistake to confuse
molarity with molality. Check your Rs and Ls!
Le Chateliers Principle
An equilibrium reaction will spontaneously balance an out-
side eect added to it. For example,
Change in amount of reactants or products: The
reaction will consume more of the substance in excess to
balance the change;
Change in volume or pressure: The reaction will form
more gas if volume increases or if pressure decreases, and
will form less gas if volume decreases or if pressure in-
creases;
Change in temperature: Endothermic reactions will
shift left for lower temperatures and shift right for higher
temperatures. Exothermic reactions will shift right for
lower temperatures and shift left for higher temperatures.
Equilibrium Constant
For reactions in a solution, the equilibrium constant of a
reaction
aA+bB
sS +tT
Is dened as
Kc =
[S]
s
[T]
t
[A]
a
[B]
b
When the reaction is in equilibrium.
If the reaction is a equilibrium between a solid and its ions
in solution, then Kc is its solubility product constant, Ksp.
If the reaction is a dissociative reaction of a weak acid, then
Kc is its acid dissociation constant, Ka. Polyprotic (hav-
ing more than one H) acids have multiple Ka, but usually
Ka1 determines the pH.
For a conjugate acid-base pair, Ka Kb = Kw.
If the reactants are gases, then the equilibrium constant is
dened as
Kp =
P
s
S
P
t
T
P
a
A
P
b
B
When the reaction is in equilibrium.
The Formulas above, when applied to non-equilibrium sit-
uations, gives Q. The reaction forms products if Q < K,
reactants if Q > K, and nothing if Q = K (already in
equilibrium).
3
Equilibrium Constant (cont.)
For all equilibrium reactions, there are more reactants than
products if K < 1, more products than reactants if K > 1,
and the same amount of reactants and products if K = 1.
Acid Character of Hydrogen Atoms
Hydrogen atoms are acidic when they are weakly bonded,
and when the molecule/atom they are bonded to forms
stable anions.
In organic compounds, the hydrogen atoms in carboxyl
groups (COOH) are usually the most acidic.
Indicators for Acid-Base Titration
Indicator Small pH Color change Large pH
Methyl violet Yellow 0.01.6 Violet
Bromophenol
blue
Yellow 3.04.6 Blue
Methyl orange Red 3.14.4 Yellow
Methyl red Red 4.4-6.2 Yellow
Litmus Red 5.08.0 Yellow
Bromothymol
blue
Yellow 6.07.6 Blue
Phenolphthalein Colorless 8.310.0 Pink
Oxidation and Reduction
Oxidation Reduction
Loss of electrons Gains electrons
Oxidation num. increases Oxidation num. decreases
Occurs at anode Occurs at cathode
Mnemonic devices:
OIL RIG (Oxidation Is Loss, Reduction Is Gain)
What an ox loses, a red cat gains (An =
anode; ox = oxidation; red = reduction; cat = cath-
ode)
Electrochemical Formulas
Electromotive force E = (G)/(nF)
Nernst equation E = E
(RT/nF) ln Q
E = E
(0.0592/n) log Q
Standard cell potential E
= E
red
cathode E
red
anode
Energy of a charged particle E = qV
Faradays Law of Electrolysis m = (Q/F)(M/z)
Types of Magnetic Materials
Paramagnetic: Can be magnetized to attract external
magnetic elds, but cannot retain magnetism. Param-
agnetic materials have a magnetic permeability of more
than 0. They usually have free electrons, especially d
and f electrons. Their magnetization follows Curies Law
(M = C B/T). Examples of paramagnets are tungsten
and cesium.
Types of Magnetic Materials (cont.)
Diamagnetic: Can be magnetized to repulse external
magnetic elds, but cannot retain magnetism. Diamag-
netic materials have a magnetic permeability of less than
0. Examples of diamagnets are bismuth and antimony.
Ferromagnetic: Can be magnetized and retain mag-
netism. Ferromagnetism depends both on the chemical
composition and the structure of the material (iron is a
ferromagnet, while stainless steel is not). Examples of fer-
romagnets include cobalt and iron.
Nuclear Chemistry
Alpha particles () Helium nuclei (
4
2
He)
Beta particles (
) Electrons (
0
1
e)
Positrons (
+
) Antielectrons (
0
1
e)
Gamma radiation () High energy radiation (
0
0
)
Units of radioactivity SI: Becquerel (Bq): 1 nucleus/s
(Disintegration per second) Curie (Ci): 3.7 10
10
nuclei/s
Units of absorbed radiation SI: Gray (Gy): 1 J/kg
(Energy per kilogram tissue) Rad: 0.01 Gy
Metallurgy
Metallurgy is the extraction of minerals from ores.
Pyrometallurgy: The use of heat to convert ores to met-
als. (Example: Production of iron)
Hydrometallurgy: The use of chemical processes in a so-
lution to separate a metal from its ore. (Example: Bayer
process for producing aluminum)
Electrometallurgy: The use of electrochemical processes
to separate a metal. (Example: Hall process for producing
aluminum)
Hydrocarbons
Name Common Formula Hybridization
Alkane CnH2n+2 sp
3
Cycloalkane CnH2n sp
3
Alkene CnH2n sp
2
Alkyne CnH2n2 sp
Aromatic CnH2n6 sp
2
In a hydrocarbon with n carbons, the number of hydrogens
is 2n + 2, minus 2 for each bond or carbon ring.
Stereoisomerism
Stereoisomerism occurs at bonds such as C=C, where both
ends have two dierent substituents, because the rotation
of these substituents are restricted.
Cis-trans isomerism: If both ends have a hydrogen
atom substituent, then the compound exhibits cis-trans
isomerism. The cis-isomer has both hydrogen atoms on the
same side, and the trans-isomer has the hydrogen atoms
on dierent sides.
Stereoisomerism (cont.)
E/Z isomerism: If the two ends of the bond do not have a
common hydrogen atom, then the compound exhibits E/Z
isomerism. The Z isomer has the larger substituents
(dened by the CIP Rules) of both ends on the same side,
while the E isomer has the larger substituents on dierent
sides.
Cahn-Ingold-Prelog Rules
The CIP Rules are used to compare two substituent groups
in the E/Z and R/S groups of naming isomers.
1. Direct comparison: If the atoms that are di-
rectly connected to the stereocenter are dierent,
then the atom with a higher atomic number receives
higher priority.
2. Tiebreaker I: If there is a tie, then a list of atoms
two bonds away from the stereocenter is compiled
for each of the two substituent groups. The atoms
with the greatest atomic number from each list are
then compared. If they tie, then the second greatest
atoms from each list are compared. This process is
repeated until the tie is broken.
3. Tiebreaker II: If there is still a tie after consider
atoms two bonds away from the center, then atoms
three bonds away are considered in the same way in
Tiebreaker I. This process is repeated until the tie is
broken.
4. Isotopes: If two groups dier only in isotopes (and
are otherwise identical), then mass number is used
instead of atomic number in the process.
5. Double and triple bonds: If there is a dou-
ble bond in the substituent group, then the dou-
ble bond is treated as a bond with ghost atoms
(e.g. R-A=B-R is treated as R-(A-B)-(B-A)-R).
Triple bonds, similarly, have two ghost atoms for
each atom.
6. Cycles: To handle a molecule containing one or
more cycles, one must rst expand it into a tree
(called a hierarchical digraph by the authors) by
traversing bonds in all possible paths starting at
the stereocenter. When the traversal encounters an
atom through which the current path has already
passed, a ghost atom is generated in order to keep
the tree nite.
4
Criteria of Aromaticity
If a hydrocarbon
1. Is cyclic, i.e. possesses a carbon ring;
2. Is planar, i.e. all carbons on the ring are on the same
plane;
3. Has an uninterrupted cloud of electrons;
4. The number of pairs of electrons in the cloud is
an odd number, i.e. the number of electrons in the
cloud is 4n + 2;
then the hydrocarbon is aromatic. Aromatic compounds
are highly stable (cannot undergo addition reactions), but
can undergo substitution reactions.
Functional Groups
Functional Group Name Sux/Prex
R-OH (hydroxyl) alcohol -ol
R-O-R (ether) ether ether
R-X (halo) haloalkane halo-
R-NH2 (amino) amine -amine
R-COH (aldehyde) aldehyde -al
R-COX (haloformyl) acyl halide -oyl halide
R-CO-R (carbonyl) ketone -one
R-COOH (carboxyl) carboxylic acid -oic acid
R-COO
(
carboxylate) carboxylate -oate
R-COO-R (ester) ester -oate
R-CONH2 (amide) amide -amide
R-CNH-R (ketimine) ketimine imino-
R-CHNH (aldimine) aldimine imino-
R-CONCO-R
(imide)
imide imido-
R-N3 (azide) azide azido-
R-N2-R (azo) azo azo-
R-OCN (cyanate) cyanate cyanato-
R-NCO (isocyanate) isocyanate isocyanato-
R-CN (nitrile) nitrile cyano-
R-NC (isonitrile) isonitrile isocyano-
R-NO (nitroso) nitroso nitroso-
R-NO2 (nitro) nitro nitro-
R-ONO (nitrosooxy) nitrite nitrosooxy-
R-ONO2 (nitrate) nitrate nitroxy-
R-SH (sulfhydryl) thiol -thiol
R-SCN (thiocyanate) thiocyanate thiocyanato-
R-NCS (isothio-
cyanate)
isothiocyanate isothiocyanato-
R-CSH (carbonoth-
ioyl)
thial -thial
R-PH3 (phosphino) phosphine -phosphane
R-C6H5 (phenyl, Ph) benzene der. phenyl-
Functional Groups (cont.)
Functional Group Name Sux/Prex
R-CH2C6H5 (benzyl,
Bn)
toluene der. benzyl-
R-C5H4N (pyridyl) pyridine der. pyridin-x-yl
Note: In actual compounds, change all instances of halo
above to halogen names (uoro, chloro, bromo, iodo).
Amino Acids
Hydrophobic amino acids:
Name Code Name Code
Alanine Ala Valine Val
Phenylalanine Phe Methionine Met
Leucine Leu Proline Pro
Isoleucine Ile Tryptophane Trp
Hydrophilic amino acids:
Name Code Name Code
Glycine Gly Threonine Thr
Serine Ser Cysteine Cys
Tyrosine Tyr Asparagine Asn
Glutamine Gln Arginine Arg
Lysine Lys Histidine His
Aspartic acid Asp Glutamic acid Glu
Protein Structure
Proteins are large biochemical complexes that contain sev-
eral polypeptide compounds (amino acid chains). They
are organized into four levels of structure:
Primary structure: The chain of amino acids that make
up the protein; this chain directly controls the other levels
of protein structure.
Secondary structure: The patterns formed by segments
of the polypeptide chain; can be either -helices or -
pleated sheets.
Tertiary structure: The folding of the polypeptide to
produce a certain shape.
Quarternary structure: The geometrical bonding of
several polypeptides to form the protein.
Chirality
A molecule possessing a nonsuperimposable mirror image
is chiral.
Two mirror images of a chiral molecule are enantiomers.
A carbon that is bonded to 4 dierent groups is an asym-
metric center. Chiral molecules have at least one asym-
metric centers.
Chiral molecules rotate polarized light. Two enantiomers
rotate polarized light by the same degrees, one clockwise
and one counterclockwise. A mixture of two enantiomers
in 1:1 does not rotate polarized light, and is racemic.
Enantiomerism
System Name Based On
R/S Structure
(+)/() Direction of rotation of polarized
light
D/L Enantiomer of glyceraldehyde the
molecule is derived from
R/S notation: Orient the enantiomer so that the small-
est (by CIP Rules) substituent points backward (away from
the viewer) and the largest substituent points upward. If
the larger substituent of the other two points toward the
right, then the enantiomer is an R-enantiomer. If the larger
substituent points toward the left, then the enantiomer is
an L-enantiomer.
(+)/() notation: An enantiomer that rotates the plane
of polarization clockwise is dextrorotary (+). An enan-
tiomer that rotates the plane of polarization counterclock-
wise is levorotary ().
D/L notation: An enantiomer that is derived from (+)-
glyceraldehyde is the D-enantiomer. An enantiomer that
is derived from ()-glyceraldehyde is the L-enantiomer.
Note that nomenclature in a system cannot be determined
by that in another system.
Caution: The (+)/() system is sometimes written as
(d)/(l), which is easily confused with the D, L system. As
these two systems sometimes conict (a D-enantiomer can
be an (l)-enantiomer), the (+)/() notations are strongly
preferred.
Signicant Figures
Signicant gures (sig gs) is the number of digits that
carry precision in a number.
Non-measured Numbers: Non-measured numbers,
such as , integer counts, denition of units, etc. always
have innite sig gs. Other constants, such as NA, have
limited sig gs.
Non-zero Digits: Nonzero digits are always signicant,
unless one or more of the other rules are violated.
Zeros: Leading zeros are never signicant; trailing zeros,
however, are signicant only if they are part of the mea-
surement. Zeros between non-zero digits are always signif-
icant.
Reporting Numbers: Reported numbers are only signif-
icant to the precision of the equipments with which they
are measured.
Addition/Subtraction: When adding or subtracting
two numbers, the result should have as many decimal
places as the number with the smallest sig gs.
Multiplication/Division: When multiplying or divid-
ing, the result should have as many sig gs as the number
with the smallest sig gs.
c 20092011 Zee Zuo. Licensed under CC-BY-SA 3.0, United States. Revision 3
5
You might also like
- Physics Final (Cheat Sheet) With ProblemsDocument2 pagesPhysics Final (Cheat Sheet) With ProblemsRSlipkov97% (97)
- Chemistry Cheat SheetDocument5 pagesChemistry Cheat Sheetdadadabababa100% (9)
- Organic Chemistry Cheat Sheet For Midterm2015 Ucsc Chem110bDocument2 pagesOrganic Chemistry Cheat Sheet For Midterm2015 Ucsc Chem110bStarrx714100% (2)
- Chemistry Study Guide/Notes For Final Exam SCH3U Grade 11Document21 pagesChemistry Study Guide/Notes For Final Exam SCH3U Grade 11Niki83% (53)
- (Tom Brody) Nutritional Biochemistry Second Editi PDFDocument1,012 pages(Tom Brody) Nutritional Biochemistry Second Editi PDFRahma Yeni100% (1)
- Physics I Final Cheat SheetDocument1 pagePhysics I Final Cheat SheetJsea2011100% (1)
- CM2192 CheAnalytical Chemistry Cheat Analytical Chemistry Cheat Sheet 2Document1 pageCM2192 CheAnalytical Chemistry Cheat Analytical Chemistry Cheat Sheet 2dorothyhuangNo ratings yet
- Sterling Test Prep College Organic Chemistry Practice Questions: Practice Questions with Detailed ExplanationsFrom EverandSterling Test Prep College Organic Chemistry Practice Questions: Practice Questions with Detailed ExplanationsNo ratings yet
- Master CheatsheetDocument7 pagesMaster Cheatsheetaquamagie50% (2)
- Analytical Chemistry Cheat SheetDocument2 pagesAnalytical Chemistry Cheat Sheetdorothyhuang100% (7)
- 86 Tricks To Ace Organic ChemistryDocument164 pages86 Tricks To Ace Organic Chemistryjinzo8892% (12)
- Chemistry Cheat SheetDocument5 pagesChemistry Cheat SheetEJ FelisildaNo ratings yet
- Chemistry Cheat SheetDocument10 pagesChemistry Cheat Sheetbrook93% (40)
- 16 - MCAT G-Chem Formula SheetDocument2 pages16 - MCAT G-Chem Formula SheetNathan Korean Kim100% (7)
- Physics Cheat Sheet MasterDocument6 pagesPhysics Cheat Sheet Masteradamhameleh100% (6)
- Organic II Reactions (Complete) BETADocument21 pagesOrganic II Reactions (Complete) BETATheoNo ratings yet
- Chemistry Final Cheat SheetDocument1 pageChemistry Final Cheat SheetScott Allred100% (2)
- Bio Cheat Sheet MasterDocument7 pagesBio Cheat Sheet MasterChris_Barber0986% (7)
- Chemistry Ion Cheat SheetDocument2 pagesChemistry Ion Cheat SheetTiffany Gallina100% (4)
- Physics Cheat SheetDocument1 pagePhysics Cheat Sheetjlvmrbd7770% (1)
- Nelson Grade 11 Chemistry ReviewDocument10 pagesNelson Grade 11 Chemistry Reviewexhalait67% (3)
- Organic Chemistry NotesDocument6 pagesOrganic Chemistry NotesVersiform89% (9)
- Summary of Important Organic ReactionsDocument41 pagesSummary of Important Organic ReactionsKathyNo ratings yet
- DAT Bootcamp - Reaction Summary Sheet PDFDocument28 pagesDAT Bootcamp - Reaction Summary Sheet PDFbbNo ratings yet
- Welcome To Enagic PresentationDocument31 pagesWelcome To Enagic PresentationTinker Bell'n100% (3)
- CHem Cheat SheetDocument8 pagesCHem Cheat SheetPaulDahlberg100% (1)
- Chemistry Cheat Sheet (2010RR)Document3 pagesChemistry Cheat Sheet (2010RR)JethroKuan100% (1)
- Chemistry Cheat SheetDocument3 pagesChemistry Cheat Sheetyash patelNo ratings yet
- O1: The Science of Chemistry 03: Basic Math For Chemistry: Chemistry Core Concept Master Cheat SheetDocument7 pagesO1: The Science of Chemistry 03: Basic Math For Chemistry: Chemistry Core Concept Master Cheat SheetGuillermo Narváez Lozano100% (1)
- OChem MasterDocument6 pagesOChem MasterDelixae PhoinixNo ratings yet
- Fundamental of Organic ChemistryDocument11 pagesFundamental of Organic ChemistryBernie Suarez50% (2)
- Cheat Sheet For Organic Chemistry Midterm 1 2015 - 1Document1 pageCheat Sheet For Organic Chemistry Midterm 1 2015 - 1Norma Leticia Ramos33% (3)
- Preliminary Instructions: Cu Ni CR Fe FeDocument4 pagesPreliminary Instructions: Cu Ni CR Fe FeEmmanuel Ryan100% (1)
- Chem Cheat Sheet MasterDocument6 pagesChem Cheat Sheet MasteradamhamelehNo ratings yet
- Organic Chemistry Summary ReactionsDocument49 pagesOrganic Chemistry Summary Reactionsjordi17100% (1)
- Chemistry Cheat SheetDocument2 pagesChemistry Cheat Sheetprincessrgl_112003705467% (3)
- CHEM Cheat Sheet 3Document2 pagesCHEM Cheat Sheet 3Ruby Rodriguez100% (2)
- Formula Sheet Physics 133Document12 pagesFormula Sheet Physics 133Muhammad IshtiaqNo ratings yet
- Precalculus NotesDocument5 pagesPrecalculus NotesMuneebKhanNo ratings yet
- Physics Cheat SheetDocument2 pagesPhysics Cheat SheetSam L'Huillier75% (4)
- Chemistry Final Exam Study GuideDocument9 pagesChemistry Final Exam Study GuideJosh MorganNo ratings yet
- Organic Chemistry IDocument10 pagesOrganic Chemistry Iscribblerofnonsense80% (5)
- Chemistry ReviewDocument3 pagesChemistry ReviewJanine100% (1)
- Organic Chemistry Past Exam KeyDocument102 pagesOrganic Chemistry Past Exam KeyParker McColl86% (7)
- Rate Law GraphsDocument2 pagesRate Law GraphsChris_Barber09No ratings yet
- Gen Chem Day 1 - The Basics, Stoichiometry, Atomic/Electronic StructureDocument17 pagesGen Chem Day 1 - The Basics, Stoichiometry, Atomic/Electronic StructureconjurerscienceNo ratings yet
- Lecture 21, Electrochemistry and ReactionsDocument40 pagesLecture 21, Electrochemistry and ReactionsHuraira Abid100% (1)
- Chem 114 Electrochemical Energy 2Document32 pagesChem 114 Electrochemical Energy 2KaizNo ratings yet
- General Chemistry ReviewDocument15 pagesGeneral Chemistry ReviewPhirun ChengNo ratings yet
- 5.3 Notes Redox EquilibriaDocument21 pages5.3 Notes Redox EquilibriaDiego Istheillest HinesNo ratings yet
- ElectrochemistryDocument2 pagesElectrochemistryotrebolNo ratings yet
- CLS JEEAD-19-20 XII Che Target-1 Level-1 Chapter-3Document26 pagesCLS JEEAD-19-20 XII Che Target-1 Level-1 Chapter-3Archita Ray0% (1)
- NOTES ElectrochemistryDocument30 pagesNOTES ElectrochemistryAlexander LeeNo ratings yet
- Periodic TableDocument28 pagesPeriodic TablegajenraoNo ratings yet
- Week 4 - Basic Concept of Corrosion - Part 1Document75 pagesWeek 4 - Basic Concept of Corrosion - Part 1Araasu EgambaramNo ratings yet
- Electro Chemistry and CorrosionDocument10 pagesElectro Chemistry and CorrosiondeeparamaniNo ratings yet
- Electrochemistry Syllabus: Sengunthar Engineering College Department of ChemistryDocument10 pagesElectrochemistry Syllabus: Sengunthar Engineering College Department of ChemistrydeeparamaniNo ratings yet
- Electrochemistry: (Tuesday, 8 May 2017)Document18 pagesElectrochemistry: (Tuesday, 8 May 2017)mipa amarNo ratings yet
- Electro Chemistry SND CorrosionDocument10 pagesElectro Chemistry SND CorrosiondrgviswaNo ratings yet
- Namma Kalvi 11th Chemistry 2 Mark and 3 Mark Notes em 216472Document16 pagesNamma Kalvi 11th Chemistry 2 Mark and 3 Mark Notes em 216472vvn natrajNo ratings yet
- 5 - Electrochemistry PDFDocument15 pages5 - Electrochemistry PDFthinkiit100% (1)
- Titration Project ReportDocument19 pagesTitration Project ReportPayal Niharika67% (3)
- Gosser D. R For Quantitative Chemistry 2023Document119 pagesGosser D. R For Quantitative Chemistry 2023jobpei2No ratings yet
- Learning Activity No. 3 Reactions of The Hydrogen Sulfide Group (Ions Are Separated As Sulfides in Their Acid Solutions)Document23 pagesLearning Activity No. 3 Reactions of The Hydrogen Sulfide Group (Ions Are Separated As Sulfides in Their Acid Solutions)sampong mga dalere100% (1)
- Water AnalysisDocument19 pagesWater AnalysisTowfiq Hossain TaskuNo ratings yet
- 3 Titrasi Asam Basa Introduction-1Document24 pages3 Titrasi Asam Basa Introduction-1sofyan novrizalNo ratings yet
- Msds Naoh 5%Document6 pagesMsds Naoh 5%Arfin Fardiansyah100% (1)
- Processes: Alkaline Water Electrolysis Powered by Renewable Energy: A ReviewDocument23 pagesProcesses: Alkaline Water Electrolysis Powered by Renewable Energy: A ReviewJoshua MitchellNo ratings yet
- Misconceptions in ChemistryDocument299 pagesMisconceptions in ChemistryRinaFaridaBuangetNo ratings yet
- Game Changer - RecordDocument3 pagesGame Changer - RecordUsman Ahmad KhanNo ratings yet
- 3-Introduction To Medicinal Chemistry-And Physicochemical PropertiesDocument39 pages3-Introduction To Medicinal Chemistry-And Physicochemical PropertiesKvvPrasadNo ratings yet
- Chemistry 9701 GCE A/AS Level For Examination in 2008Document70 pagesChemistry 9701 GCE A/AS Level For Examination in 2008Hubbak KhanNo ratings yet
- 2.pH, Buffers and Isotonic Solutions AbDocument48 pages2.pH, Buffers and Isotonic Solutions AbPasham Venkat ReddyNo ratings yet
- Final Chemistry IADocument13 pagesFinal Chemistry IASanjai AnanthNo ratings yet
- Lab chm301 Carboxylic AcidDocument7 pagesLab chm301 Carboxylic AcidbbbbNo ratings yet
- Chemistry WorksheetDocument6 pagesChemistry WorksheetRashida TahaNo ratings yet
- Fiveable AP Chem Cram ChartDocument1 pageFiveable AP Chem Cram ChartkeipherNo ratings yet
- Goc 02 SheetDocument62 pagesGoc 02 SheetmikcNo ratings yet
- Acid Bases and Salts Worksheet 1Document6 pagesAcid Bases and Salts Worksheet 1Pooja Debnath100% (3)
- Chapter 05 PDFDocument10 pagesChapter 05 PDFHamid Zahoor100% (1)
- 102 AbrevsDocument7 pages102 AbrevsHandugan Quinlog NoelNo ratings yet
- Science QuizDocument32 pagesScience QuizSamineni JaneNo ratings yet
- Heat of NeutralisationDocument4 pagesHeat of NeutralisationZarith Hidayah IsmileNo ratings yet
- Chemistry (Chemical Energy) PDFDocument3 pagesChemistry (Chemical Energy) PDFMohammad RussellNo ratings yet
- C2 Chemistry Revision PosterDocument5 pagesC2 Chemistry Revision PosterАня ИвановаNo ratings yet
- Section: Site 2Document5 pagesSection: Site 2Hubbak KhanNo ratings yet
- Assignment Chem 353.1 TU Attempt ReviewDocument16 pagesAssignment Chem 353.1 TU Attempt ReviewJason SummatNo ratings yet
- Red Valve - Caustic SlurryDocument2 pagesRed Valve - Caustic SlurryJane MuderNo ratings yet
- Delhi Public School Bulandshahr: Class X Holidays Homework PhysicsDocument12 pagesDelhi Public School Bulandshahr: Class X Holidays Homework PhysicsHarshit GoelNo ratings yet