Professional Documents
Culture Documents
Study Guide
Study Guide
Uploaded by
Vasin Dumrongprechachan0 ratings0% found this document useful (0 votes)
10 views1 pageA solution consists of a solute dissolved in a solvent, with the solvent being the most abundant component. The physical state of the solvent usually determines the physical state of the solution. Solubility is defined as the maximum amount of solute that can dissolve in a fixed amount of solvent at a given temperature when excess solute is present. Miscible liquids form homogeneous solutions by mixing in all proportions, while the principle of "like dissolves like" means substances with similar intermolecular forces can dissolve each other.
Original Description:
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA solution consists of a solute dissolved in a solvent, with the solvent being the most abundant component. The physical state of the solvent usually determines the physical state of the solution. Solubility is defined as the maximum amount of solute that can dissolve in a fixed amount of solvent at a given temperature when excess solute is present. Miscible liquids form homogeneous solutions by mixing in all proportions, while the principle of "like dissolves like" means substances with similar intermolecular forces can dissolve each other.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageStudy Guide
Study Guide
Uploaded by
Vasin DumrongprechachanA solution consists of a solute dissolved in a solvent, with the solvent being the most abundant component. The physical state of the solvent usually determines the physical state of the solution. Solubility is defined as the maximum amount of solute that can dissolve in a fixed amount of solvent at a given temperature when excess solute is present. Miscible liquids form homogeneous solutions by mixing in all proportions, while the principle of "like dissolves like" means substances with similar intermolecular forces can dissolve each other.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
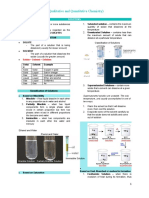
CHM 116 Study Guide: Properties of Solution Basics about solutions Solute = the substance that dissolves in a solvent
Solvent = the most abundant component - The physical state of solvent usually determines the physical state of solvent. Solubility (S) = the maximum amount that dissolves in a fixed quantity of a given solvent at a given temperature, where an excess of the solute is present. Miscibility = the property of liquids to mix in all proportions, forming a homogeneous solution. Like dissolves like = substance with similar types of intermolecular forces dissolve in each other.
You might also like
- Physics (Electric Flux)Document3 pagesPhysics (Electric Flux)Charline Lucille BanoNo ratings yet
- Chemistry (Solutions)Document3 pagesChemistry (Solutions)Charline Lucille BanoNo ratings yet
- 4th Qtr. Module Week 4Document3 pages4th Qtr. Module Week 4Avirel Reynante PodadorNo ratings yet
- Saturated and UnsaturatedDocument20 pagesSaturated and UnsaturatedBernadette Sta. AnaNo ratings yet
- Is Matter Around Us Pure Notes PDFDocument15 pagesIs Matter Around Us Pure Notes PDFgkclubakshayaNo ratings yet
- Gen ChemDocument2 pagesGen ChemTango Jhecee Meir, D.No ratings yet
- UntitledDocument1 pageUntitledYyan TadeoNo ratings yet
- Solubility of Drugs PDFDocument66 pagesSolubility of Drugs PDFPrabhas MeherNo ratings yet
- SolutionsDocument32 pagesSolutionsMayuresh PanseNo ratings yet
- Solubility and Distribution Phenomena: Aseel SamaroDocument89 pagesSolubility and Distribution Phenomena: Aseel Samaroveneta gizdakovaNo ratings yet
- 2.solutions FDocument33 pages2.solutions Fshrutianand8915No ratings yet
- Solubility and Distribution PhenomenaDocument89 pagesSolubility and Distribution Phenomenadesekar sejati100% (2)
- CHEM 2 Digital Note Taking TemplateDocument1 pageCHEM 2 Digital Note Taking Templatekasandra cristy galonNo ratings yet
- Solubility Notes Summary Physical Pharmacy PharmaceuticsDocument9 pagesSolubility Notes Summary Physical Pharmacy PharmaceuticsYuppie RajNo ratings yet
- GenChem2 ReviewerDocument11 pagesGenChem2 ReviewerJules Kirsten RueloNo ratings yet
- Solubility of DrugsDocument147 pagesSolubility of Drugsharshagadia234No ratings yet
- SOLUTIONSDocument15 pagesSOLUTIONSdivinegrace.cruz.mnlNo ratings yet
- Physical Properties of Solution: Group 1Document27 pagesPhysical Properties of Solution: Group 1Althea BacordoNo ratings yet
- Gen. Chem 2 ReviewerDocument2 pagesGen. Chem 2 ReviewerAshley NicoleNo ratings yet
- Physical Properties of SolutionDocument39 pagesPhysical Properties of SolutionAlice RiveraNo ratings yet
- ChemistryDocument3 pagesChemistryJared AlexanderNo ratings yet
- Solutions: Physical Properties - OFDocument4 pagesSolutions: Physical Properties - OFChristina RañaNo ratings yet
- Hypotonic Because It Has Less of The Solute Molecules, But More of TheDocument1 pageHypotonic Because It Has Less of The Solute Molecules, But More of TheCaitlyn9777No ratings yet
- 12stem B - Week7Document3 pages12stem B - Week7Franz SorianoNo ratings yet
- SolutionsDocument17 pagesSolutionsNursherina AsanjiNo ratings yet
- SolutionsDocument32 pagesSolutionsAditya PandeyNo ratings yet
- CH 2Document8 pagesCH 2akshat bhardwajNo ratings yet
- Science 7 - SolutionsDocument12 pagesScience 7 - SolutionsKirk KinoNo ratings yet
- Solubility in Inorganic ChemistryDocument17 pagesSolubility in Inorganic ChemistryGessa GelocaNo ratings yet
- CHEM - Molecular View of The Solution ProcessDocument10 pagesCHEM - Molecular View of The Solution Processlux luciNo ratings yet
- SolutionsDocument32 pagesSolutionsdeepbag79No ratings yet
- LAS 1.2 MixturesDocument1 pageLAS 1.2 Mixturesxhiriii mindzzNo ratings yet
- SolubilityDocument59 pagesSolubilityNadem DreemNo ratings yet
- Learning Material 2: General Chemistry 2 PLM For February 26-March 5,2021Document5 pagesLearning Material 2: General Chemistry 2 PLM For February 26-March 5,2021Justeny TabbayNo ratings yet
- CBSE Class 12 Chemistry Quick Revision Notes Solutions: Material Downloaded From SUPERCOPDocument5 pagesCBSE Class 12 Chemistry Quick Revision Notes Solutions: Material Downloaded From SUPERCOPNothing is ImpossibleNo ratings yet
- Chapter 16 SolutionsDocument11 pagesChapter 16 SolutionsMarco VillarosaNo ratings yet
- 6.P.2.3 - SolubilityDocument9 pages6.P.2.3 - SolubilityCinta KimiaNo ratings yet
- Chapter 12: Solutions: 12.1 Setting The Stage: Some Important TerminologyDocument5 pagesChapter 12: Solutions: 12.1 Setting The Stage: Some Important TerminologyCarlos Mella-RijoNo ratings yet
- Analytical Chemistry (Qualitative and Quantitative Chemistry)Document6 pagesAnalytical Chemistry (Qualitative and Quantitative Chemistry)Rizza OlivaNo ratings yet
- 2 SolutionsDocument5 pages2 SolutionsPreeti AgrawalNo ratings yet
- Science q1 w3 Factors Affecting SolubilityDocument20 pagesScience q1 w3 Factors Affecting SolubilityMallen MallenNo ratings yet
- L 3 - SolutionsDocument51 pagesL 3 - SolutionsJayRiveraNo ratings yet
- Orca Share Media1601430625143 6716886876762277304Document16 pagesOrca Share Media1601430625143 6716886876762277304Neil MaglalangNo ratings yet
- Intermolecular Forces of Attraction (IMFA)Document3 pagesIntermolecular Forces of Attraction (IMFA)Anne Gelli ManaloNo ratings yet
- Unit - I: Solubility of Drugs: Mahatma Gandhi Institute of Pharmacy, LucknowDocument23 pagesUnit - I: Solubility of Drugs: Mahatma Gandhi Institute of Pharmacy, LucknowMukesh TiwariNo ratings yet
- Lesson No.4: Inorganic Chemistry (Sci Ac5)Document5 pagesLesson No.4: Inorganic Chemistry (Sci Ac5)Franklin BayaniNo ratings yet
- Holiday HomeworkDocument10 pagesHoliday HomeworkAnushree TiwariNo ratings yet
- Module 2 SolutionDocument2 pagesModule 2 SolutionLJ Valdez100% (1)
- SolutionsDocument32 pagesSolutionsdebrishibanerjee125No ratings yet
- Chapter 3 - Solutions and Solution PreparationDocument35 pagesChapter 3 - Solutions and Solution Preparationbahru demekeNo ratings yet
- Lecture Notes 4: Colligative Properties: ΔG = Δh ‐ TδsDocument14 pagesLecture Notes 4: Colligative Properties: ΔG = Δh ‐ TδsIwanNo ratings yet
- Solutions - SolubilityDocument16 pagesSolutions - SolubilityÖmer KhanNo ratings yet
- Classifying SolutionsDocument17 pagesClassifying Solutionsapi-307707832No ratings yet
- Chemistry Midterm Study GuideDocument9 pagesChemistry Midterm Study GuiderachelelizabethNo ratings yet
- Chemistry +2hhsliveDocument76 pagesChemistry +2hhsliveNaVaneeth :/No ratings yet
- Solution CDocument14 pagesSolution CPankaj SawantNo ratings yet
- Class Xii 1,2,3 SolutionDocument50 pagesClass Xii 1,2,3 SolutionSubhasish SauNo ratings yet
- Solubility: Francisco, Angelica Garcia, Drexler Gopez, Jewel Guieb, Luichito Halon, Mikaela ErikaDocument10 pagesSolubility: Francisco, Angelica Garcia, Drexler Gopez, Jewel Guieb, Luichito Halon, Mikaela ErikaHannah Valerie NuqueNo ratings yet
- The Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksFrom EverandThe Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksNo ratings yet
- Vedic Wisdom: Selected verses from the vedas for material gain and spiritual happinessFrom EverandVedic Wisdom: Selected verses from the vedas for material gain and spiritual happinessRating: 4.5 out of 5 stars4.5/5 (12)