Professional Documents
Culture Documents
كتاب الكيمياء للصف السادس العلمي الأحيائي 2016 29
كتاب الكيمياء للصف السادس العلمي الأحيائي 2016 29
Uploaded by
Ahmad SadounOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
كتاب الكيمياء للصف السادس العلمي الأحيائي 2016 29
كتاب الكيمياء للصف السادس العلمي الأحيائي 2016 29
Uploaded by
Ahmad SadounCopyright:
Available Formats
-22680 J
= q = mol J/ 1334118 -
0.017 mol
:
2-1 H = qP = - 1334118 J/ mol
g 3
(( )N H
2 4
J kJ . mol J/
)32 g/mole : kJ
g 1000
(1 )kJ
(J/g.C ( )4.2 ) ( ) (
H kJ/mol = H J/ mol
(1000 )J
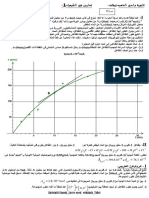
24.6C
.28.2C
(1 )kJ
(H )kJ/mol( = - 1334118 )J/ mol
(1000 )J
1 mole = - 1334 kJ/mol
kJ/mol
:
.
- 161 kJ/mol : . - 1334 kJ/mol
10-1
(
. )
: 1 -
.
.
:
(H2O)s (H2O)l H = 6 kJ/mol
kJ/mol 6
25C atm .1
:)(
29
You might also like
- تمارين الطاقة الداخلية مع التصحيح PDFDocument13 pagesتمارين الطاقة الداخلية مع التصحيح PDFHadir kahoul100% (7)
- 3as U01 - E3 - Cour-Exe 01Document37 pages3as U01 - E3 - Cour-Exe 01abdelhalimNo ratings yet
- مثال الانبوبة الشعريةDocument2 pagesمثال الانبوبة الشعريةكلية الهندسة - محاضرNo ratings yet
- 2AS-019 - موضوع اختبار تجريبيDocument6 pages2AS-019 - موضوع اختبار تجريبيAmir OuerdiNo ratings yet
- 1AS U07 - E3 - Cour-Exe 04Document6 pages1AS U07 - E3 - Cour-Exe 04Kaddache CharidNo ratings yet
- 3AS U01 - E3 - Cour-Exe 01 PDFDocument33 pages3AS U01 - E3 - Cour-Exe 01 PDFnehal niniNo ratings yet
- Comp3Sec SalahDocument2 pagesComp3Sec SalahmutraceNo ratings yet
- الفرض 2 PDFDocument2 pagesالفرض 2 PDFbrini medNo ratings yet
- البولي بروبلينDocument20 pagesالبولي بروبلينabdlrahman56k78No ratings yet
- 11 Medical PhysicsDocument24 pages11 Medical PhysicsAbdo MoatyNo ratings yet
- Physics Se Bac2012 CorrectionDocument2 pagesPhysics Se Bac2012 Correctionkhamis farid0% (1)
- سلسلة تمارين الاحماض و الاسس و الاكسدة و الارجاعDocument18 pagesسلسلة تمارين الاحماض و الاسس و الاكسدة و الارجاعzaza madjbNo ratings yet
- كتابي ج01Document100 pagesكتابي ج01Boualem LaichaouiNo ratings yet
- الفرض الجهوي لاولمبياد الفيزياء و الكيمياء 2013 ، 2014Document6 pagesالفرض الجهوي لاولمبياد الفيزياء و الكيمياء 2013 ، 2014Sayf EDDINENo ratings yet
- 03 Sujets de Concours D'accès Au Doctorat en Physique Energétique - Ouargla 2021Document11 pages03 Sujets de Concours D'accès Au Doctorat en Physique Energétique - Ouargla 2021Ahmed BelguenouneNo ratings yet
- فروض الفيزياء والكيمياء الثانية باك علوم فيزيائية الدورة الاولى المرحلة 2 النموذج 12 غ.مDocument2 pagesفروض الفيزياء والكيمياء الثانية باك علوم فيزيائية الدورة الاولى المرحلة 2 النموذج 12 غ.مYuta HaiseNo ratings yet
- Cmp2Sec AbbasDocument1 pageCmp2Sec AbbasmutraceNo ratings yet
- Exoch 3Document1 pageExoch 3الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- d8b6 FinDocument2 pagesd8b6 FinSiMo ElbNo ratings yet
- 2 5402080015162545523Document131 pages2 5402080015162545523حسنين فلاح حسن جاسمNo ratings yet
- الفرض الجهوي لاولمبياد الفيزياء و الكيمياء 2011 ، 2012)Document6 pagesالفرض الجهوي لاولمبياد الفيزياء و الكيمياء 2011 ، 2012)Sayf EDDINENo ratings yet
- Dev211pr ZeghdoudDocument1 pageDev211pr ZeghdoudFahim TighemineNo ratings yet
- كيمياء حركية PDFDocument294 pagesكيمياء حركية PDFسعدNo ratings yet
- ملزمة حركية التفاعلات الكيميائية 1 المرحلة الثالثة كيمياءDocument35 pagesملزمة حركية التفاعلات الكيميائية 1 المرحلة الثالثة كيمياءmahmoudNo ratings yet
- Phys - Chem Work2 Lectrur 5Document66 pagesPhys - Chem Work2 Lectrur 5melad syNo ratings yet
- ملخص الوحدة 6Document4 pagesملخص الوحدة 6Massi HsnNo ratings yet
- سلسلة تمارين في الديناميككا الحراريةDocument13 pagesسلسلة تمارين في الديناميككا الحراريةamineNo ratings yet
- أفكار الباب الثالثDocument10 pagesأفكار الباب الثالثYoussef AboagagaNo ratings yet
- نشاط 14- المواصلة والموصليةDocument1 pageنشاط 14- المواصلة والموصليةmohamed safiNo ratings yet
- التعويض النيوكلوفيليDocument21 pagesالتعويض النيوكلوفيليNadherdaman AlshamaryNo ratings yet
- M Eg H: Na2CO3Document5 pagesM Eg H: Na2CO3samirus incNo ratings yet
- DS2 1 Bac 2014Document1 pageDS2 1 Bac 2014Youssef HalloumiNo ratings yet
- 2-05-1 - تعيين كمية المادة عن طريق قياس الناقليةDocument34 pages2-05-1 - تعيين كمية المادة عن طريق قياس الناقليةInda MineNo ratings yet
- السادس العلمي الفصل الاول الاستاذ احمد النداوي PDFDocument58 pagesالسادس العلمي الفصل الاول الاستاذ احمد النداوي PDFCanYa DigItNo ratings yet
- استهلاك المادة العضويةDocument25 pagesاستهلاك المادة العضويةAbdessamad NfissiNo ratings yet
- d8b1d982d985 2 D8a7d98ad8a9 D8a7d8a6Document2 pagesd8b1d982d985 2 D8a7d98ad8a9 D8a7d8a6مهدي محمدNo ratings yet
- EI2prem NGDocument3 pagesEI2prem NGbouthyyNo ratings yet
- EI2prem NGDocument3 pagesEI2prem NGSafa MamacheNo ratings yet
- - نماذج احيائي منهج عربي 4Document4 pages- نماذج احيائي منهج عربي 4hassanghfran283No ratings yet
- 3Document6 pages3samirus incNo ratings yet
- ��كيم 214 معدلة 2023�Document129 pages��كيم 214 معدلة 2023�Fatima Ishaq AlfatlawiNo ratings yet
- $RRIOBB8Document4 pages$RRIOBB8pelkis08No ratings yet
- Exoch 1Document2 pagesExoch 1الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- DS5R 2bac 2014 PCDocument4 pagesDS5R 2bac 2014 PCMouhibi AbdellahNo ratings yet
- تمارين 4 التناقص الإشعاعيDocument3 pagesتمارين 4 التناقص الإشعاعيRachid SadNo ratings yet
- الحجم المولي للغازاتDocument14 pagesالحجم المولي للغازاتKorichiKarimNo ratings yet
- Composition de Physique 3AS - Sujet 01Document2 pagesComposition de Physique 3AS - Sujet 01Hasan Rajawi100% (1)
- M (CHO) 90 G.molDocument2 pagesM (CHO) 90 G.molMedjkoune NabilNo ratings yet
- 2Document60 pages2hadeelk822No ratings yet
- 5-Noyaux, Masse, EnergieDocument7 pages5-Noyaux, Masse, EnergieFarida LatracheNo ratings yet
- سلسلة تمارين المكتسبات القبلية 2022Document7 pagesسلسلة تمارين المكتسبات القبلية 2022Miss MNo ratings yet
- Ku-18888 2 PDFDocument10 pagesKu-18888 2 PDFAli WaliNo ratings yet
- 1694521730Document22 pages1694521730bkr664868No ratings yet
- Doc8 U1Document2 pagesDoc8 U1blue and grayNo ratings yet
- SERIEDocument2 pagesSERIESoukainaNo ratings yet
- سلسلة تمارين في الديناميككا الحرارية مع الحلولDocument12 pagesسلسلة تمارين في الديناميككا الحرارية مع الحلولAiglecity OukassouNo ratings yet
- Partie Théorique TP Chimie Des SurfacesDocument9 pagesPartie Théorique TP Chimie Des SurfacesMebarka TimNo ratings yet
- اختبار مصححDocument3 pagesاختبار مصححmutraceNo ratings yet