Professional Documents
Culture Documents
Ammonium Hydroxide Material Balance
Uploaded by
Jishnu John0 ratings0% found this document useful (0 votes)
21 views3 pagesIt is a material and energy balance of ammonia done using excel.
Copyright
© © All Rights Reserved
Available Formats
XLSX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentIt is a material and energy balance of ammonia done using excel.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
21 views3 pagesAmmonium Hydroxide Material Balance
Uploaded by
Jishnu JohnIt is a material and energy balance of ammonia done using excel.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
You are on page 1of 3
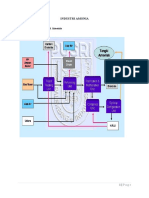
Basis: 1000 kg of Ammonium Hydroxide
25°C Air 13.29646 kmol
Manufacturing of
1atm (Nitrogen) 10.5042 kmol
VAHP
ΔQ=0
25°C
1atm Oxygen
Nitrogen 10.5042 kmol Nitrogen
Oxygen 2.792256 kmol Hydrogen
457°C Hydrogen 31.51261 kmol
1atm 457°C
1atm
Carbon Dioxide 7.878151 kmol
COMBUSTION
CHEMBER
ΔH°f=
Natural Gas 8.292791 kmol
Steam 16.58558 kmol
25°C
1atm
Manufacturing of Ammonium Hydroxide
Assuming no pressure correction in th
the reactor. Assuming the value of pre
400°C
1atm
2.792256 kmol
10.5042 kmol REACTOR
31.51261 kmol [Fe catalyst]
400-450°C
200atm
ΔH°f=
Nitrogen 0.205882 kmol
Hydrogen 0.617647 kmol 450°C
Ammonia 20.58824 kmol 1atm
Oxygen 2.792256 kmol
Nitrogen 0.205882 kmol
Hydrogen 0.617647 kmol
CHILLER
ΔQ=
Ammonia 350 kg 32°C
35% (by mass) 1atm
Water
650 kg AGITATOR
ΔH°f=
Ammonium Hydroxide 1000 kg
no pressure correction in the reactor
or. Assuming the value of pressure to be 1atm.
You might also like
- Chemistry School Center by SlidesgoDocument48 pagesChemistry School Center by SlidesgoRoy JekriNo ratings yet
- Calculation Title: Seagas Pipeline Design - MinervaDocument1 pageCalculation Title: Seagas Pipeline Design - Minerva秦东旺No ratings yet
- Neraca Massa AmmoniakDocument11 pagesNeraca Massa AmmoniakAnnisa ShafiraNo ratings yet
- Neraca Massa AmmoniakDocument10 pagesNeraca Massa AmmoniakMuhammad FadilNo ratings yet
- Calculate Boiler Efficiency Using Direct MethodDocument53 pagesCalculate Boiler Efficiency Using Direct Methoddineshkbunker08No ratings yet
- Qu. All - NDocument28 pagesQu. All - NArima KouseiNo ratings yet
- CP DT: Gas Butano (Entrada)Document3 pagesCP DT: Gas Butano (Entrada)Diego TavizónNo ratings yet
- Slip Calcluation in Ammonia PlantDocument8 pagesSlip Calcluation in Ammonia PlantRajat Chauhan0% (1)
- Material Balance NaOH PlantDocument8 pagesMaterial Balance NaOH Plantpankaj verma100% (4)
- Lampiran A Sudah FinalDocument20 pagesLampiran A Sudah FinalBayu Handika PrasetyoNo ratings yet
- Combustion CalcsDocument8 pagesCombustion CalcsZhaqir HusseinNo ratings yet
- Combustion CalcsDocument8 pagesCombustion Calcs31331311313No ratings yet
- Aspen Exchanger Design and Rating Fired Heater V11Document1 pageAspen Exchanger Design and Rating Fired Heater V11MarcoNo ratings yet
- Double Pipe Heat Exchanger Analysis (Example 5.1) : Water WaterDocument3 pagesDouble Pipe Heat Exchanger Analysis (Example 5.1) : Water WaterBagusRekaNo ratings yet
- CO2 Capture from Coal Power Plants Using Amine AbsorptionDocument23 pagesCO2 Capture from Coal Power Plants Using Amine AbsorptionGhochapon MongkhonsiriNo ratings yet
- Komponen BM (Kg/kmol) INPUT (KG/HR)Document11 pagesKomponen BM (Kg/kmol) INPUT (KG/HR)AchmadJa'farShodiqShahabNo ratings yet
- Boiler efficiency and flue gas analysis calculationsDocument50 pagesBoiler efficiency and flue gas analysis calculationsDilip MishraNo ratings yet
- Chlorine_Material-2520BalanceDocument5 pagesChlorine_Material-2520BalanceAditya KumarNo ratings yet
- Line 1 Calculation REV 1 PDFDocument6 pagesLine 1 Calculation REV 1 PDFYYON KYNN KOHNo ratings yet
- Thermal System Analysis Document TitleDocument4 pagesThermal System Analysis Document TitleAisyah NandanitaNo ratings yet
- Heat BalanceDocument33 pagesHeat BalanceIrshad Hussain100% (2)
- ChE 111P: Heat and mass balances of humidification and condensation processesDocument6 pagesChE 111P: Heat and mass balances of humidification and condensation processesMateo PremarionNo ratings yet
- Emission Calculation 2Document12 pagesEmission Calculation 2myungkwan haNo ratings yet
- AppendixDocument51 pagesAppendixgemilang perdikaNo ratings yet
- Jawaban SlideDocument3 pagesJawaban SlideCitra SalbellaNo ratings yet
- Feed flow rate and F-T reactor analysisDocument7 pagesFeed flow rate and F-T reactor analysismahmoudNo ratings yet
- Combustion CalculationDocument16 pagesCombustion Calculationmohamed Elsayed0% (1)
- Heat Balance-1Document85 pagesHeat Balance-1Ravi sharmaNo ratings yet
- Thermophysical PropertiesDocument7 pagesThermophysical PropertiesWei JianNo ratings yet
- Gas StoichiometryDocument10 pagesGas StoichiometryAnn DayritNo ratings yet
- Experiment - 7: Aim: Sizing of Pressure VesselDocument5 pagesExperiment - 7: Aim: Sizing of Pressure VesselHomesick TutorsNo ratings yet
- 13 46 PDFDocument2 pages13 46 PDFjhomalyn mae alsolaNo ratings yet
- 13 46 PDFDocument2 pages13 46 PDFjhomalyn mae alsolaNo ratings yet
- 13 46Document2 pages13 46Апцгдк Ьфш БгднчллNo ratings yet
- 13 46Document2 pages13 46Hawraa AlbahadlyNo ratings yet
- 13 46 PDFDocument2 pages13 46 PDFDavid GaviolaNo ratings yet
- CHE 482 Reaction Engineering & Separations Including Properties Abdulaziz Oqlah Abdullah AbdulraheemHamad Alhajri Ali AlsubaieDocument11 pagesCHE 482 Reaction Engineering & Separations Including Properties Abdulaziz Oqlah Abdullah AbdulraheemHamad Alhajri Ali AlsubaieTimelessNo ratings yet
- Effect of False Air On Heat Consumption: Note Change Values Only in Shaded CellsDocument7 pagesEffect of False Air On Heat Consumption: Note Change Values Only in Shaded Cellshmaza shakeelNo ratings yet
- PIPE Constants and ConversionsDocument2 pagesPIPE Constants and ConversionsJames Joseth ArceoNo ratings yet
- Chem Notes 10,11-3,4Document6 pagesChem Notes 10,11-3,4delacruzmamikaelaNo ratings yet
- Raw Mill-Ball Mill Heat BalanceDocument16 pagesRaw Mill-Ball Mill Heat BalanceAbhishekNo ratings yet
- Heat Exchanger Design CalculationsDocument8 pagesHeat Exchanger Design Calculationskikokiko KarimNo ratings yet
- Plant InvesticationDocument3 pagesPlant InvesticationirfanNo ratings yet
- Analytical Data: Table 1: Basic Crude Oil Analysis For Sample Collected From Well # H-18 Experiment Method ResultDocument2 pagesAnalytical Data: Table 1: Basic Crude Oil Analysis For Sample Collected From Well # H-18 Experiment Method ResultYousef Adel HassanenNo ratings yet
- Specific Gas Ratio - SwapnilDocument33 pagesSpecific Gas Ratio - SwapnilYhane100% (1)
- Material and Energy Balance of Urea Reactor and Stripper Saipem ProcessDocument20 pagesMaterial and Energy Balance of Urea Reactor and Stripper Saipem ProcessBalas43100% (1)
- Lampiran IIDocument30 pagesLampiran IIyogaNo ratings yet
- Energy Balance For E-102: Stream 7 200°C and 2 Atm Stream 8 410°C and 2atmDocument5 pagesEnergy Balance For E-102: Stream 7 200°C and 2 Atm Stream 8 410°C and 2atmAhmed Qutb AkmalNo ratings yet
- AcetaldehydeDocument98 pagesAcetaldehydeKrishna DangiNo ratings yet
- (Supercritical Unit) Date: 02.05.2011: 1 X 660 MW TPP For Visa Power Limited at RaigarhDocument4 pages(Supercritical Unit) Date: 02.05.2011: 1 X 660 MW TPP For Visa Power Limited at RaigarhirfanNo ratings yet
- Neraca Masa Dan GPMDocument8 pagesNeraca Masa Dan GPMAdam RizkyNo ratings yet
- BT FCC CNLD2Document8 pagesBT FCC CNLD2ncn1812No ratings yet
- 3-3. Material and Energy Balance:: AssumptionsDocument55 pages3-3. Material and Energy Balance:: AssumptionsbommaNo ratings yet
- CO2 Plant DesignDocument12 pagesCO2 Plant DesignOmprakaash MokideNo ratings yet
- Gas Laws KEYDocument2 pagesGas Laws KEYKeNo ratings yet
- MASS BALANCE FOR ACRYLIC ACID PRODUCTIONDocument6 pagesMASS BALANCE FOR ACRYLIC ACID PRODUCTIONHaziq AzliNo ratings yet
- Analytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryFrom EverandAnalytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryNo ratings yet
- FLOW THROUGH FLUIDIZED BED LABDocument2 pagesFLOW THROUGH FLUIDIZED BED LABJishnu JohnNo ratings yet
- Manufacturing Industry PPT Template 0001Document3 pagesManufacturing Industry PPT Template 0001Jishnu JohnNo ratings yet
- B M W M: IO Edical Aste AnagementDocument23 pagesB M W M: IO Edical Aste AnagementJishnu JohnNo ratings yet
- Foggler 4 Solved Problem Using ExcelDocument672 pagesFoggler 4 Solved Problem Using ExcelJishnu JohnNo ratings yet
- Natural Gas Processing - Summary & IntroductionDocument7 pagesNatural Gas Processing - Summary & IntroductiondndudcNo ratings yet
- SWECs External 15 June 2020Document230 pagesSWECs External 15 June 2020Hamzah ZakiNo ratings yet
- Hydrocracker Complex Presentation For BPST 23Document50 pagesHydrocracker Complex Presentation For BPST 23fbriandityaNo ratings yet
- Calculating CP: Temperature Range and FormulasDocument2 pagesCalculating CP: Temperature Range and Formulas김일태No ratings yet
- SUEZ's Water and Process Solutions For The Chemical IndustryDocument5 pagesSUEZ's Water and Process Solutions For The Chemical IndustrymnasiroleslamiNo ratings yet
- HidrogenoDocument24 pagesHidrogenoDaniel Rodriguez santanderNo ratings yet
- MSENSE® DGA 9 Technical Data 03 2021 enDocument4 pagesMSENSE® DGA 9 Technical Data 03 2021 enTa Huy CuongNo ratings yet
- EIL - HSE Bulletin No. 1 - Liquefied Petroleum Gas (LPG) - Prepared by Mr. G Shashidhar, Manager (HSEMS)Document1 pageEIL - HSE Bulletin No. 1 - Liquefied Petroleum Gas (LPG) - Prepared by Mr. G Shashidhar, Manager (HSEMS)rajaguru20003No ratings yet
- Sustainable Construction SolutionsDocument71 pagesSustainable Construction SolutionsArunashish MazumdarNo ratings yet
- 2017-04 Clean Agent Fire Extinguishing SystemsDocument11 pages2017-04 Clean Agent Fire Extinguishing Systemsahtin618No ratings yet
- Welding Calculator BOHLERDocument7 pagesWelding Calculator BOHLERKhamdi AfandiNo ratings yet
- WGM GasTech Presentation PDFDocument25 pagesWGM GasTech Presentation PDFariel100% (1)
- O-ring material and size guideDocument247 pagesO-ring material and size guideGustavoNo ratings yet
- BIOMASS GASIFICATION PROCESS OVERVIEWDocument23 pagesBIOMASS GASIFICATION PROCESS OVERVIEWbharatNo ratings yet
- Sonatrach: JOB NO.: J6404Document56 pagesSonatrach: JOB NO.: J6404Rabah AmidiNo ratings yet
- Role of Chemical Engineers in Present ScenarioDocument19 pagesRole of Chemical Engineers in Present Scenariosamvendan100% (1)
- Endress-Hauser Analog Chlorine Dioxide Sensor CCS50 ENDocument3 pagesEndress-Hauser Analog Chlorine Dioxide Sensor CCS50 ENTrân Nguyễn Đoàn QuếNo ratings yet
- CH 4034 Comprehensive Design Project II Interim Report 1: Production of Ammonia From NaphthaDocument21 pagesCH 4034 Comprehensive Design Project II Interim Report 1: Production of Ammonia From NaphthaAlfonso BlancoNo ratings yet
- Chemsheets GCSE 1112 Gas Volumes 2Document1 pageChemsheets GCSE 1112 Gas Volumes 2Enlai RooneyNo ratings yet
- Chemical Plant Utility - Nitrogen System DesignDocument10 pagesChemical Plant Utility - Nitrogen System DesignIJRASETPublicationsNo ratings yet
- Haber ProcessDocument19 pagesHaber ProcessLauren CaseNo ratings yet
- Effects of Globalization on Indian Industries and Impact on Key SectorsDocument35 pagesEffects of Globalization on Indian Industries and Impact on Key Sectorsvivek14d89No ratings yet
- A Comparison of Physical Solvents For Acid Gas Removal PDFDocument10 pagesA Comparison of Physical Solvents For Acid Gas Removal PDFVirnia PatziNo ratings yet
- Safe Use of Cylinder Gases: ©consultnet LimitedDocument29 pagesSafe Use of Cylinder Gases: ©consultnet LimitedAhmad Mensa100% (1)
- Chapter 4 - StudentDocument69 pagesChapter 4 - Studenteja70No ratings yet
- Upgrade Your Air Preheater with BD Heat RecoveryDocument1 pageUpgrade Your Air Preheater with BD Heat RecoveryAssure TurbinesNo ratings yet
- Nitrogen RejectionDocument7 pagesNitrogen RejectionOmar TocmoNo ratings yet
- Crossword ExamDocument1 pageCrossword Examtwilight_6teenNo ratings yet
- DETCON Product GuideDocument56 pagesDETCON Product GuideSergio HernandezNo ratings yet
- Empirical Carbon Isotope/maturity Relationships For Gases From Algal Kerogens and Terrigenous Organic Matter, Based On Dry, Open-System PyrolysisDocument9 pagesEmpirical Carbon Isotope/maturity Relationships For Gases From Algal Kerogens and Terrigenous Organic Matter, Based On Dry, Open-System PyrolysisPracoyo Adi PNo ratings yet