Professional Documents
Culture Documents
Carnot Engine (Application of The Second Law of Thermodynamics) Problems and Solutions PDF
Uploaded by
FUN SCIENCEOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carnot Engine (Application of The Second Law of Thermodynamics) Problems and Solutions PDF
Uploaded by
FUN SCIENCECopyright:
Available Formats
https://physics.gurumuda.
net
Carnot engine (application of the second law of thermodynamics) - problems and
solutions
1. An engine operates between 1200 Kelvin and 300 Kelvin. What is the efficiency of this engine ?
Known :
High temperature (TH) = 1200 K
Low temperature (TL) = 300 K
Wanted : Efficiency (e)
Solution :
T −TL
e= H
TH
1200− 300 900
e= = =0.75
1200 1200
75
e= =75
100
2. An engine operates between 727oC and 127oC. The engine's heat input is 6000 Joule. What is the
efficiency of the engine and work done by the engine each cycle.
Known :
High temperature (TH) = 727oC + 273 = 1000 K
Low temperature (TL) = 127oC + 273 = 400 K
Heat input (QH) = 6000 Joule
Wanted : Efficiency (e) and work done by the engine (W)
Solution :
Efficiency of the Carnot engine :
T −TL
e= H
TH
1000− 400 600
e= = =0.60
1000 1000
60
e= =60
100
Work is done by the Carnot engine :
W = e QH
W = (0.6)(6000)
W = 3600 Joule
3. An engine operates between 527oC and 127oC. The engine's heat input is 10,000 Joule. How
much heat is discharged as waste heat from this engine ?
Known :
High temperature (TH) = 527oC + 273 = 800 K
Low temperature (TL) = 127oC + 273 = 400 K
Heat input (QH) = 10,000 Joule
Wanted : Heat output (QL)
Solution :
Efficiency of the carnot engine :
© 2018 | San Alexander
https://physics.gurumuda.net
TH−TL
e=
TH
800 −400 400
e= = =0.5
800 800
Work is done by Carnot engine :
W = e Q1
W = (0.5)(10,000)
W = 5000 Joule
Heat output (QL) = Heat input (QH) – Work (W)
QL = Q H – W
QL = 10,000 – 5,000
QL = 5000 Joule
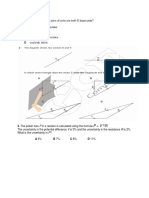
4. What is the efficiency of the engine and work done by the engine according to diagram below.
Known :
High temperature (TH) = 800 K
Low temperature (TL) = 300 K
Heat input (QH) = 1000 Joule
Wanted : Efficiency (e) and work (W) done by the engine
Solution :
Efficiency of Carnot engine :
T −TL
e= H
TH
800 −300 500
e= = =0.625
800 800
62.5
e= =62.5
100
Work is done by the Carnot engine :
W = e QH
W = (0.625)(1000)
W = 625 Joule
© 2018 | San Alexander
You might also like
- Thermal Properties of Matter-1Document25 pagesThermal Properties of Matter-1HariNo ratings yet
- Physics GRE Solutions OmnibusDocument561 pagesPhysics GRE Solutions OmnibusTaylor Faucett100% (6)
- CHAPTER 1 Design Criteria - General Requirements Rev 0Document26 pagesCHAPTER 1 Design Criteria - General Requirements Rev 0saminaali100% (3)
- Claus Borgnakke, Richard E. Sonntag-Fundamentals of Thermodynamic, Instructor Solution PDFDocument3,302 pagesClaus Borgnakke, Richard E. Sonntag-Fundamentals of Thermodynamic, Instructor Solution PDFNNNNo ratings yet
- Physics EquationsDocument5 pagesPhysics Equationsanon-992211100% (64)
- Supplementary Problems:: Mechanical Engineering DepartmentDocument10 pagesSupplementary Problems:: Mechanical Engineering DepartmentJhun BrionesNo ratings yet
- Thermodynamics IDocument7 pagesThermodynamics IJustinnNo ratings yet
- Astig M.E.Document21 pagesAstig M.E.Aj Buniag100% (1)
- Fluids HW SolutionDocument8 pagesFluids HW SolutionDennis Brown100% (1)
- Ipe Plate 2 Fluid MachineriesDocument93 pagesIpe Plate 2 Fluid Machineriesjanuel borelaNo ratings yet
- Ther 3Document11 pagesTher 3albert nicolasNo ratings yet
- 8.1 Differential Equations 01 SolutionsDocument5 pages8.1 Differential Equations 01 SolutionsmaverNo ratings yet
- (Solved Problems) : Thermodynamics 01Document6 pages(Solved Problems) : Thermodynamics 01Ben0% (1)
- Dual Cycles LessonDocument17 pagesDual Cycles LessonMae Anj100% (1)
- Problem Set PPE Day 5Document4 pagesProblem Set PPE Day 5Reinzo GallegoNo ratings yet
- CombinepdfDocument108 pagesCombinepdfAlessandra TanNo ratings yet
- ETest - Lezioni Di Fisica TecnicaDocument208 pagesETest - Lezioni Di Fisica TecnicaJack FlashNo ratings yet
- Chapter 1 Jan2013Document30 pagesChapter 1 Jan2013enteryourname5No ratings yet
- Jugo Chapter 12Document21 pagesJugo Chapter 12Reinzo Gallego100% (1)
- Applied Mechanical Engineering Engine Power Cycle: SolutionDocument6 pagesApplied Mechanical Engineering Engine Power Cycle: SolutionAlteaAlNo ratings yet
- TP0000065 (E) Platinum RTDs PDFDocument17 pagesTP0000065 (E) Platinum RTDs PDFCarlosNo ratings yet
- ReviewerDocument8 pagesReviewermaylynXiXNo ratings yet
- Carnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsDocument2 pagesCarnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsFUN SCIENCENo ratings yet
- Carnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsDocument2 pagesCarnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsFUN SCIENCENo ratings yet
- Chapter 04 The Second Law of Thermodynamics (PP 82-99)Document18 pagesChapter 04 The Second Law of Thermodynamics (PP 82-99)Muhammad Ashfaq Ahmed71% (7)
- Thermo1 (20162)Document37 pagesThermo1 (20162)hillary100% (2)
- Refrigeration Engineering: MerefengDocument62 pagesRefrigeration Engineering: MerefengPrecious Gallardo DerainNo ratings yet
- Concept Question IPEDocument65 pagesConcept Question IPEAnne Gabrielle DavidNo ratings yet
- A Reheat Cycle With Two Stage of Reheating Is Executed With Steam Expanding Initially From 90 Bar and 530c The Two Reheater Pressure Are 10 BarDocument1 pageA Reheat Cycle With Two Stage of Reheating Is Executed With Steam Expanding Initially From 90 Bar and 530c The Two Reheater Pressure Are 10 BarJerico Andrada50% (4)
- Compilation of Solved Sample Problems in Power Plant EngineeringDocument38 pagesCompilation of Solved Sample Problems in Power Plant EngineeringAnthropophobe NyctophileNo ratings yet
- ThermodynamicsDocument2 pagesThermodynamicsEduNo ratings yet
- ThermodynamicsDocument57 pagesThermodynamicsMei Lamfao100% (1)
- Refrigeration Problem SetsDocument5 pagesRefrigeration Problem SetsNiño Gerard JabagatNo ratings yet
- Prelim Quizzes To Midterms Heat TransferDocument11 pagesPrelim Quizzes To Midterms Heat TransferNiño Gerard JabagatNo ratings yet
- Experiment 2 HORSEPOWER EFFICIENCY GEAR RATIO AND SPEED RATIODocument10 pagesExperiment 2 HORSEPOWER EFFICIENCY GEAR RATIO AND SPEED RATIOJake Polo SantiagoNo ratings yet
- Hydraulic AnsweranssDocument11 pagesHydraulic AnsweranssJanelle D. Puti-anNo ratings yet
- ME REVIEW ThermodynamicsDocument65 pagesME REVIEW ThermodynamicsKhate ÜüNo ratings yet
- Introduction To ThermodynamicsDocument66 pagesIntroduction To ThermodynamicsMikaela Dela Cruz0% (1)
- CHAPTER 5, Nunbers 1 To 5Document3 pagesCHAPTER 5, Nunbers 1 To 5Shane Buraga67% (3)
- 19 21Document6 pages19 21Aub Enriquez100% (1)
- Gas Cycle 2 Otto CycleDocument3 pagesGas Cycle 2 Otto CycleRonnieNo ratings yet
- Problem Set PPE Day 6Document3 pagesProblem Set PPE Day 6Reinzo GallegoNo ratings yet
- Isobaric ProcessDocument24 pagesIsobaric ProcessWhindy Bagawisan CasugaNo ratings yet
- A Single Acting Compressor Has A Volumetric Efficiency of 87Document2 pagesA Single Acting Compressor Has A Volumetric Efficiency of 87Harley Quinn0% (1)
- Hea RansferDocument40 pagesHea RansferHikki KunNo ratings yet
- (X3a) Activity 1 - 2 CompressorDocument6 pages(X3a) Activity 1 - 2 CompressorLester Alfred M. OlasimanNo ratings yet
- Estrada, Chapter 4Document14 pagesEstrada, Chapter 4Reinzo GallegoNo ratings yet
- Machine Design II: Engr. Zhierwinjay M. Bautista Name of FacultyDocument11 pagesMachine Design II: Engr. Zhierwinjay M. Bautista Name of FacultyJOSEPH REFUERZONo ratings yet
- AlgebraDocument7 pagesAlgebrarochelleNo ratings yet
- D. Deg R 1.8 Deg K: Page 1 of 14Document14 pagesD. Deg R 1.8 Deg K: Page 1 of 14Joseph BallenaNo ratings yet
- Pipe ProbsDocument6 pagesPipe ProbsTrecia Ann Combalicer HuploNo ratings yet
- Mikee EncodedDocument2 pagesMikee EncodedjaysonNo ratings yet
- 2.0 Band Brake Example ProblemsDocument10 pages2.0 Band Brake Example Problemsmanalo.jonmeloNo ratings yet
- Reviewer in Heat TransferDocument12 pagesReviewer in Heat TransferKristian Allen B. DomingoNo ratings yet
- ICE - Lecture From MapuaDocument48 pagesICE - Lecture From MapuaMarcial Jr. MilitanteNo ratings yet
- Refrigeration Systems: Ice Refrigeration and Product Load RefrigerationDocument14 pagesRefrigeration Systems: Ice Refrigeration and Product Load RefrigerationCllyan Reyes100% (1)
- Heat Transfer: Precious Arlene Villaroza-MelendrezDocument46 pagesHeat Transfer: Precious Arlene Villaroza-MelendrezMark Jake Rodriguez0% (1)
- Donguiz, Shiela MareDocument12 pagesDonguiz, Shiela Mareyeng botzNo ratings yet
- Properties of FluidsDocument57 pagesProperties of FluidsPatricia SorianoNo ratings yet
- BSME ME122 MidtermDocument30 pagesBSME ME122 MidtermAnjo Jose JasarenoNo ratings yet
- Sentence A Is CorrectDocument14 pagesSentence A Is CorrectLister NambatacNo ratings yet
- Thermo Solutions - Part14 PDFDocument1 pageThermo Solutions - Part14 PDFLiz ArfinNo ratings yet
- Handouts PPE Day 3Document4 pagesHandouts PPE Day 3terrence miguel balitaNo ratings yet
- ME 22 (Industrial Plant Engineering) : Capitol UniversityDocument14 pagesME 22 (Industrial Plant Engineering) : Capitol UniversityBensoyNo ratings yet
- Ppe - HBW Set 3Document1 pagePpe - HBW Set 3Ben100% (1)
- Ompad Ipe 02 Prob.8Document3 pagesOmpad Ipe 02 Prob.8Sam Ompad100% (1)
- Cuison Chapter 2Document41 pagesCuison Chapter 2Reinzo GallegoNo ratings yet
- Bsme 3-B: Me 114 - Heat Transfer Bachelor of Science in Mechanical EngineeringDocument3 pagesBsme 3-B: Me 114 - Heat Transfer Bachelor of Science in Mechanical EngineeringJethro Briza GaneloNo ratings yet
- Carnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsDocument1 pageCarnot Engine (Application of The Second Law of Thermodynamics) Problems and SolutionsBasic PhysicsNo ratings yet
- GRE Physics Test: Practice BookDocument91 pagesGRE Physics Test: Practice BookGalo CandelaNo ratings yet
- Study SheetDocument14 pagesStudy SheetHari BilasiNo ratings yet
- Pgre NotesDocument111 pagesPgre Notest ElderNo ratings yet
- Flexible Magnetic Filaments and Their ApplicationsDocument13 pagesFlexible Magnetic Filaments and Their ApplicationsFUN SCIENCENo ratings yet
- Politecnico Di Milano Novel Technique For Dic Speckle Pattern Optimization and Generation PDFDocument127 pagesPolitecnico Di Milano Novel Technique For Dic Speckle Pattern Optimization and Generation PDFFUN SCIENCENo ratings yet
- IBPS RRB Syllabus 2020 For Prelims & Mains, Download PDFDocument1 pageIBPS RRB Syllabus 2020 For Prelims & Mains, Download PDFFUN SCIENCENo ratings yet
- Study and Generation of Optimal Speckle Patterns For DIC: January 2007Document8 pagesStudy and Generation of Optimal Speckle Patterns For DIC: January 2007FUN SCIENCENo ratings yet
- 4 Matter WavesDocument5 pages4 Matter WavesJulian David Henao EscobarNo ratings yet
- Chapter 5 Quantum MechanicsDocument29 pagesChapter 5 Quantum MechanicsFUN SCIENCENo ratings yet
- Relativity Notes PDFDocument8 pagesRelativity Notes PDFসায়ন চক্রবর্তীNo ratings yet
- Install Guide PDFDocument176 pagesInstall Guide PDFKaren PantojaNo ratings yet
- MypttDocument1 pageMypttFUN SCIENCENo ratings yet
- ICSE Selina Solution For Class 9 Chemistry Chapter 7 Exercise QuestionsDocument31 pagesICSE Selina Solution For Class 9 Chemistry Chapter 7 Exercise QuestionsAmit100% (1)
- Chapter 1Document27 pagesChapter 1Handoko PhandaNo ratings yet
- Catálogo MK 2011/2013Document243 pagesCatálogo MK 2011/2013Grupo PriluxNo ratings yet
- StudentDocument25 pagesStudentL Michelle MackNo ratings yet
- JTM K 07Document11 pagesJTM K 07Stiven Giraldo NuñezNo ratings yet
- Electrical Engineering - Remote SensingDocument43 pagesElectrical Engineering - Remote SensingAnaStairsNo ratings yet
- PEDUCA, JoshuaDocument6 pagesPEDUCA, Joshuayeng botzNo ratings yet
- Task 2Document2 pagesTask 2Robin AdolfNo ratings yet
- Research MethodologyDocument3 pagesResearch MethodologyShailee Mehta100% (7)
- Hys007107 MS6514Document12 pagesHys007107 MS6514Avs ElectronNo ratings yet
- Poisson TermodinamicaDocument97 pagesPoisson TermodinamicaJoshua WrightNo ratings yet
- Term 1 7th MathsDocument47 pagesTerm 1 7th MathsKutty Pupps688No ratings yet
- Introduction and Basic Concepts: Dr. Javed.M ChatthaDocument32 pagesIntroduction and Basic Concepts: Dr. Javed.M ChatthaShahmirBalochNo ratings yet
- Fluid Mechanics - Lecture Notes - SCI 3114Document84 pagesFluid Mechanics - Lecture Notes - SCI 3114Sharlene Cecil PagoboNo ratings yet
- Scari de TemperaturaDocument25 pagesScari de Temperaturajo_rz_57No ratings yet
- Fundamentals of Physics Sixth Edition: Halliday Resnick WalkerDocument4 pagesFundamentals of Physics Sixth Edition: Halliday Resnick WalkerAhmar KhanNo ratings yet
- The Nature of Matter: Matter Is Made of Tiny Particles in Constant MotionDocument23 pagesThe Nature of Matter: Matter Is Made of Tiny Particles in Constant MotionMiriam QuanNo ratings yet
- Fema - 1989 Handbook of Chemical Hazard Analysis ProceduresDocument520 pagesFema - 1989 Handbook of Chemical Hazard Analysis ProceduresMario RodriguezNo ratings yet
- Thermometry Physics A LevelDocument16 pagesThermometry Physics A LevelNayana GaleaNo ratings yet
- MCQ Phy Test 1Document4 pagesMCQ Phy Test 1Aleem AhmedNo ratings yet
- AdadDocument31 pagesAdadRoge DianNo ratings yet