Professional Documents
Culture Documents
Noriel P. Padilla II 10 - Einstein Gas Laws Examples in Real Life
Uploaded by
Ashley CastroOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Noriel P. Padilla II 10 - Einstein Gas Laws Examples in Real Life
Uploaded by
Ashley CastroCopyright:
Available Formats
Noriel P.
Padilla II
10 - Einstein
Gas Laws Examples in Real Life
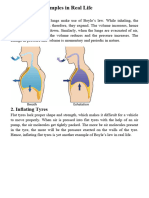
Boyle's Law - The bubbles exhaled by a scuba diver grow as the approach the surface
of the ocean. The deeper you go in the ocean the higher the pressure becomes so the
closer you are to the surface the lesser the pressure.
Charles's Law - A football inflated inside and then taken outdoors on a winter day
shrinks slightly. The inside of a house is warmer than outside on a winter day.
Gay-Lussac's Law - the shooting of a gun. As gunpowder burns, it creates superheated
gas, which forces the bullet out of the gun barrel following Gay-Lussac's Law. Other
everyday life examples can be found in things that use gas and pressure in order to
function.
Avogadro's Law - A flat tire takes up less space than an inflated tire.
Lungs expand as they fill with air. Exhaling decreases the volume of the lungs.
A balloon filled with helium weighs much less than an identical balloon filled with
air.
Combined Gas Law - an example of this is a combustion engine. The engine takes in
fuel(volume) ignites it which increases the temperature which then creates pressure
which pushes the piston of the engine making it run.
Ideal Gas Law - The high pressured gas tanks, cannisters and cisterns. That is why a
typical cistern, used for gas transportation, carries the gasses in highly compressed
states, because if you put the gas into the cannister under high pressure, you can carry
around gasses in huge quantities
You might also like
- Compilation OF Different Gas LawsDocument7 pagesCompilation OF Different Gas LawsJean Angelove SantosNo ratings yet
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Charles LawDocument12 pagesCharles LawLerr Real RelleNo ratings yet
- GENCHEM 1 - Week 5 - Activity 8Document1 pageGENCHEM 1 - Week 5 - Activity 8Contreras JerwhinNo ratings yet
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Gas LawsDocument20 pagesGas Lawsapi-305343335No ratings yet
- Applications of Charles LawDocument2 pagesApplications of Charles LawHelmaNo ratings yet
- 3 Theories of Gas LawsDocument14 pages3 Theories of Gas LawsLindsey YntasNo ratings yet
- Austin, Vinny, Steven - Combined Gas Law PresentationDocument24 pagesAustin, Vinny, Steven - Combined Gas Law PresentationAustin MaNo ratings yet
- Different Laws of Chemistry and Their Day ToDocument40 pagesDifferent Laws of Chemistry and Their Day ToSahejNo ratings yet
- Charles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayDocument2 pagesCharles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayEnriquez JohnNo ratings yet
- Special Cases of The Ideal Gas LawDocument8 pagesSpecial Cases of The Ideal Gas LawJohn Benedick Beri BernalNo ratings yet
- Scuba Diving and Gas LawDocument17 pagesScuba Diving and Gas LawTie Ing HuiNo ratings yet
- Researcher Made Test On Gas LawsDocument9 pagesResearcher Made Test On Gas LawsMaxiNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Uses and Applications of The Different Gas LawsDocument8 pagesUses and Applications of The Different Gas LawsWin7Addict100% (30)
- Use The Law To Explain The Following ObservationsDocument6 pagesUse The Law To Explain The Following ObservationsCedric Adrian AblogNo ratings yet
- Chem Law LawDocument2 pagesChem Law LawFaye Lianne MaderoNo ratings yet
- Document About Literature and HistoryDocument2 pagesDocument About Literature and HistoryMarieta GonzalesNo ratings yet
- Charles LawDocument18 pagesCharles LawfurshetnuNo ratings yet
- Chem ProjectDocument10 pagesChem Projectapi-305331033No ratings yet
- Activities in Science 10 For 4th GradingDocument2 pagesActivities in Science 10 For 4th GradingAE LLANo ratings yet
- Science 10Document6 pagesScience 10Andy ValdezNo ratings yet
- Candle Experiment UnmaskedDocument13 pagesCandle Experiment UnmaskedVijay TrivediNo ratings yet
- Gas Law: Boyle'S Law, Charles Law, Gay-Lussac'S Law. Combined Gas Law, and Ideal Gas LawDocument91 pagesGas Law: Boyle'S Law, Charles Law, Gay-Lussac'S Law. Combined Gas Law, and Ideal Gas LawLee MedalloNo ratings yet
- Fizik Charles LawDocument3 pagesFizik Charles LawDavid KhorNo ratings yet
- Ideal Gas Law: Prepared By: Kristine Hazel W. Bidayan Special Science Teacher IDocument29 pagesIdeal Gas Law: Prepared By: Kristine Hazel W. Bidayan Special Science Teacher IThami CalaguiNo ratings yet
- Gas Laws ThermodynamicsDocument27 pagesGas Laws Thermodynamicsasparomaxine2No ratings yet
- Gas Laws: Solids LiquidsDocument2 pagesGas Laws: Solids LiquidsRaymond CabanillaNo ratings yet
- What Is A Practical Application of BoyleDocument3 pagesWhat Is A Practical Application of BoyleArmstrong BosantogNo ratings yet
- Practical ApplicationsDocument3 pagesPractical ApplicationsELvin Jay CasiñoNo ratings yet
- Untitled DocumentDocument6 pagesUntitled DocumentChukwuemeka OkoyeNo ratings yet
- Gideon Elisha A. Ballesteros 10-WisdomDocument1 pageGideon Elisha A. Ballesteros 10-WisdomRandomStuffNo ratings yet
- CharlessDocument2 pagesCharlessRevilloNo ratings yet
- Charles SDocument2 pagesCharles SRevilloNo ratings yet
- How A Hot Air Balloon Works and Its Major PartsDocument1 pageHow A Hot Air Balloon Works and Its Major PartsDarknessNo ratings yet
- Emerald Green and Gold Elegant Applicant Cover LetterDocument4 pagesEmerald Green and Gold Elegant Applicant Cover LetterSam Yvhanne Quinzon TanNo ratings yet
- The Gas LawsDocument2 pagesThe Gas LawsFleur AmoureuxNo ratings yet
- GAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidDocument9 pagesGAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidMichiiee BatallaNo ratings yet
- Gas LawsDocument3 pagesGas LawsZynn Margarette DenostaNo ratings yet
- Gay-Lussac's Law or Amonton's LawDocument3 pagesGay-Lussac's Law or Amonton's LawRevilloNo ratings yet
- Avogadro's Law and Its Real Life ApplicationDocument2 pagesAvogadro's Law and Its Real Life ApplicationKaye Barrozo0% (1)
- Applications For Ideal Gas LawsDocument6 pagesApplications For Ideal Gas Lawsdreamxtreme16No ratings yet
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Chap 13Document34 pagesChap 13Iván MartinezNo ratings yet
- Title: Archimedes Blimp: Experiment DesignDocument5 pagesTitle: Archimedes Blimp: Experiment DesignTanthree QyubhyNo ratings yet
- LawDocument6 pagesLawJaymart BobilesNo ratings yet
- SciDocument2 pagesSciErica BiancaNo ratings yet
- General Chemistry I.COMBINEDGASLAWDocument12 pagesGeneral Chemistry I.COMBINEDGASLAWJennie Grace MaloomNo ratings yet
- Kinetic Molecular TheoryDocument4 pagesKinetic Molecular TheoryG-Ann N. BorjaNo ratings yet
- Boyle's LawDocument24 pagesBoyle's LawRalph Jasil Esparagoza EscanillasNo ratings yet
- Combined Gas LawDocument5 pagesCombined Gas LawjalibicharityNo ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- Avogadro's Law: States That The Volume of A Gas Is Directly Proportional To TheDocument2 pagesAvogadro's Law: States That The Volume of A Gas Is Directly Proportional To TheVñ ÕãdNo ratings yet
- Marvels of Scientific Invention An Interesting Account in Non-technical Language of the Invention of Guns, Torpedoes, Submarine Mines, Up-to-date Smelting, Freezing, Colour Photography, and many other recent Discoveries of ScienceFrom EverandMarvels of Scientific Invention An Interesting Account in Non-technical Language of the Invention of Guns, Torpedoes, Submarine Mines, Up-to-date Smelting, Freezing, Colour Photography, and many other recent Discoveries of ScienceNo ratings yet
- BehaviorofGases3 3Document7 pagesBehaviorofGases3 3Melanie TrinidadNo ratings yet
- 4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' LawsDocument54 pages4Q W1 Kinetic Molecular Theory of Gases, Boyle's and Charles' Lawsjia aganaNo ratings yet
- Gay Lussac'S Law: May Fritzi M. SaligumbaDocument2 pagesGay Lussac'S Law: May Fritzi M. SaligumbaShelly Ryn SaligumbaNo ratings yet
- Handover Analysis of 3G NetworkDocument43 pagesHandover Analysis of 3G NetworkMohammed NazimNo ratings yet
- case study-سيزمDocument51 pagescase study-سيزمHesham ElsayedNo ratings yet
- Annual Performance Accomplishment ReportDocument6 pagesAnnual Performance Accomplishment ReportJerom CanayongNo ratings yet
- Ficha Técnica Flanger y Tapas de 80cm-Peligro Alta TensiónDocument2 pagesFicha Técnica Flanger y Tapas de 80cm-Peligro Alta TensiónLeidyNo ratings yet
- Nabl 174Document34 pagesNabl 174Ashish DubeyNo ratings yet
- Introduction To Political AnalysisDocument12 pagesIntroduction To Political AnalysisAilene SimanganNo ratings yet
- ERIKS - Y Filter 1610 - Data SheetDocument1 pageERIKS - Y Filter 1610 - Data SheetNoparit KittisatitNo ratings yet
- Manual Balanza VIBRA PDFDocument89 pagesManual Balanza VIBRA PDFCalidad LassNo ratings yet
- Chapter Vocabulary Review: Name Class DateDocument1 pageChapter Vocabulary Review: Name Class Datesamixox singsNo ratings yet
- Social Media Pitch Proposal - PublicisDocument42 pagesSocial Media Pitch Proposal - PublicisЕлизаветаNo ratings yet
- Step-By-Step Guide To Essay WritingDocument13 pagesStep-By-Step Guide To Essay WritingHuynhGiangNo ratings yet
- Midea Modularni Cilleri MGB-service ManualDocument119 pagesMidea Modularni Cilleri MGB-service ManualGermánCastiglioniNo ratings yet
- Lecture No 12 MaintainabilityDocument25 pagesLecture No 12 MaintainabilityAltamash MunirNo ratings yet
- Required: If Green Machine Company Used The FIFO and The Weighted Average Methods ofDocument6 pagesRequired: If Green Machine Company Used The FIFO and The Weighted Average Methods ofrook semayNo ratings yet
- Winstar Display Co., LTD: SpecificationDocument24 pagesWinstar Display Co., LTD: SpecificationElvis SilvaNo ratings yet
- Pet Handbook Reading WritingDocument12 pagesPet Handbook Reading WritingPhương Nguyễn NguyênNo ratings yet
- The Development of Coping Resources in Adulthood: Carolyn M. Aldwin and Karen J. SuttonDocument36 pagesThe Development of Coping Resources in Adulthood: Carolyn M. Aldwin and Karen J. SuttonZuluaga LlanedNo ratings yet
- SR2019-06 (RD) Atlas of Siliceous Hot Spring Deposits - FINALDocument62 pagesSR2019-06 (RD) Atlas of Siliceous Hot Spring Deposits - FINALMichael Platas100% (1)
- MBEH'S - Final - Work (1) 27Document72 pagesMBEH'S - Final - Work (1) 27Fon Palverd Brent BridenNo ratings yet
- DLL - Mathematics 6 - Q3 - W7Document12 pagesDLL - Mathematics 6 - Q3 - W7Gerome JutajeroNo ratings yet
- OCR Physics A: 12.3 The Young Double-Slit Experiment Teacher and Technician NotesDocument5 pagesOCR Physics A: 12.3 The Young Double-Slit Experiment Teacher and Technician NotessciencedocsmanNo ratings yet
- XiGo Note 112 Pharma and The Acorn AreaDocument3 pagesXiGo Note 112 Pharma and The Acorn Areaprakush01975225403No ratings yet
- PR2 Questionnaire FinalDocument4 pagesPR2 Questionnaire FinalGevin DuetchNo ratings yet
- 7-Development of High Precision Gear MeasuringDocument9 pages7-Development of High Precision Gear MeasuringFahrul Chayank AisyahNo ratings yet
- Urban, City, and Town Planning Integrates Land Use Planning and Transportation Planning To Improve TheDocument37 pagesUrban, City, and Town Planning Integrates Land Use Planning and Transportation Planning To Improve TheMary Chriss ArnaizNo ratings yet
- SEAD - Solution Manual - 2019-2020 EditionDocument37 pagesSEAD - Solution Manual - 2019-2020 EditionYokaNo ratings yet
- Ai Lect3 Search2Document135 pagesAi Lect3 Search2Menna SaedNo ratings yet
- Reflection Activty 3 (Done)Document1 pageReflection Activty 3 (Done)theressa reidNo ratings yet
- Monologue Pirmojo Poros Kandidato VilniusDocument2 pagesMonologue Pirmojo Poros Kandidato VilniusGintarė ŽindulienėNo ratings yet
- Summer Training Report ON Analysis of Financial Trends of S.J.V.N.Ltd. (SHIMLA)Document14 pagesSummer Training Report ON Analysis of Financial Trends of S.J.V.N.Ltd. (SHIMLA)Pushp Raj ThakurNo ratings yet