Professional Documents
Culture Documents
The Boiling Points of Solutions:: Is The Molar Heat of Vaporization of The Solvent at The Specified
Uploaded by
tpjoshi1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Boiling Points of Solutions:: Is The Molar Heat of Vaporization of The Solvent at The Specified
Uploaded by
tpjoshi1Copyright:
Available Formats
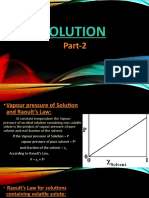
The Boiling Points of Solutions:
Solution has a lower freezing point than the pure solvent, provided no solute
separates in the solid phase, so it has a higher boiling point, provided the
solute is non-volatile and hence is not present in the vapour phase. The
problem of the variation of the boiling point of a solution with composition,
at constant pressure, may be treated by means of equations already derived,
provided the solution is dilute enough for the solvent to behave ideally. If the
solute is non-volatile, as mentioned earlier in this book, the vapour consists
entirely of solvent molecules, and hence 𝑁1′ is unity.
The equation related to boiling point of solution is mentioned below
𝑑 ln N1 ∆𝐻𝑣
( 𝑑𝑇
) = 𝑅𝑇 2
----- (1)
𝑃
Where ΔHv is the molar heat of vaporization of the solvent at the specified
temperature and pressure. By assuming this quantity to remain constant in a
small temperature range, integration of equation (1) gives
∆𝐻𝑣 𝑇 − 𝑇𝑜
ln N1 = – ( ) ----- (2)
𝑅 𝑇 𝑇𝑜
Where T is the boiling-point of the solution and To is that of the pure solvent
at the same pressure. If the system does not contain an inert gas this constant
pressure is that of the atmosphere, for the temperature is then the normal
boiling point. Because N1 is always less than unity, lnN1 is negative, and
hence T – To must be positive; consequently, the boiling point of the solution
is greater than that of the solvent. The presence of a non-volatile solute thus
raises the boiling point.
If the rise of boiling point T – To is represented by θ, it follows that for a
dilute solution,
∆𝐻𝑣
ln N1 = – 𝜃 ----- (3)
𝑅𝑇𝑜2
𝑅𝑇𝑜2
For a very dilute solution, θ = N2 ----- (4)
∆𝐻𝑣
θ ≈ λm ----- (5)
𝑅𝑇𝑜2 𝑀1
The molal boiling point elevation constant λ is equal to where ΔHv
1000∆𝐻𝑣
is the molar heat of vaporization at the boiling point To of the pure solvent, of
formula weight M1. The results given above may be used for determining the
molecular weight of a solute from the rise of boiling point of the solution.
You might also like
- Linear Algebra Cheat SheetDocument2 pagesLinear Algebra Cheat SheetBrian WilliamsonNo ratings yet
- SolutionDocument41 pagesSolutionHasib AhmedNo ratings yet
- Colligative Properties and Determination of Molar MassesDocument7 pagesColligative Properties and Determination of Molar MassesRoshini FelixNo ratings yet
- Key Concepts: TotalDocument18 pagesKey Concepts: TotalSachin Kumar67% (3)
- 1.solution and Colligative PropertiesDocument6 pages1.solution and Colligative Propertiesjch131760No ratings yet
- Lecture - 5 - Module 1Document17 pagesLecture - 5 - Module 1Zaira Eliz GonzalesNo ratings yet
- Module 1 Lecture 5Document17 pagesModule 1 Lecture 5Amirs AmjadNo ratings yet
- Changes in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventDocument5 pagesChanges in Vapour Pressure. (Vapour Pressure Lowering) : V - P Depends Only On The SolventMarthy DayagNo ratings yet
- Chapter 13 Lecture-2Document10 pagesChapter 13 Lecture-2Çetin UzişNo ratings yet
- Abstract - Freezing Point Depression Is ADocument5 pagesAbstract - Freezing Point Depression Is AMinahNo ratings yet
- Chemistry InvestigatoryDocument16 pagesChemistry InvestigatorySai NandhaNo ratings yet
- Solution (Part 2)Document24 pagesSolution (Part 2)saptarshi senNo ratings yet
- 2001 SolutionDocument12 pages2001 SolutionSaghar FaridNo ratings yet
- Lecture31 Wed Nov 29Document3 pagesLecture31 Wed Nov 29Akib ImtihanNo ratings yet
- Revised SolutionDocument42 pagesRevised SolutionRSLNo ratings yet
- SOLUTIONSDocument20 pagesSOLUTIONSnayararehman41No ratings yet
- Meeting 6-7Document30 pagesMeeting 6-7Aldo FirmansyahNo ratings yet
- Faculty: MMIK Solution Batch: 182Document6 pagesFaculty: MMIK Solution Batch: 182Md KhanNo ratings yet
- Class 12 Chemistry One LinerDocument4 pagesClass 12 Chemistry One Lineranoopkumar8127798122No ratings yet
- Type of SolutionsDocument8 pagesType of SolutionsbharathNo ratings yet
- Revision Notes On SolutionDocument8 pagesRevision Notes On SolutionSUSHMANo ratings yet
- Wa0245 1Document45 pagesWa0245 1lm7032478No ratings yet
- Arindam Das Bs-15 253 B.SC Chemistry Honors Supervised By:-Dr. S MuniDocument25 pagesArindam Das Bs-15 253 B.SC Chemistry Honors Supervised By:-Dr. S MuniArindam DasNo ratings yet
- 61 The Principles of Chemical Equil Halaman 263 288-17-26Document10 pages61 The Principles of Chemical Equil Halaman 263 288-17-26ALLIKANo ratings yet
- SolutionsDocument9 pagesSolutionsKota Venkata SukumarNo ratings yet
- Bigi Complete SolutionDocument19 pagesBigi Complete SolutionDivyanshu SharmaNo ratings yet
- Chemistryinvestigatory 160111064423Document25 pagesChemistryinvestigatory 160111064423patelalok20082006No ratings yet
- Chapter 11properties of SolutionDocument5 pagesChapter 11properties of SolutionKevin HuangNo ratings yet
- Liquid Solution (13th)Document19 pagesLiquid Solution (13th)Raju SinghNo ratings yet
- Liquid SolutionDocument16 pagesLiquid SolutionRaju SinghNo ratings yet
- PE258 Lecture 6Document21 pagesPE258 Lecture 6obumaradonaNo ratings yet
- SolutionsDocument24 pagesSolutionsSRILAKSHMI K sNo ratings yet
- Sifat Koligatif LarutanDocument28 pagesSifat Koligatif LarutanDiah SukmawatiNo ratings yet
- 4 Colligativeproperties 180825211533Document88 pages4 Colligativeproperties 180825211533Rmon ianNo ratings yet
- ColligativeDocument24 pagesColligativeHarry WinstonNo ratings yet
- 4 Colligative Properties of SolutionsDocument84 pages4 Colligative Properties of SolutionsujargohandiNo ratings yet
- Unit 9 Lecture Day 4-Colligative PropertiesDocument41 pagesUnit 9 Lecture Day 4-Colligative PropertiesPutri Nur AuliyaNo ratings yet
- Learning Materials of Chemistry For Board ExamDocument80 pagesLearning Materials of Chemistry For Board Examnwork0274No ratings yet
- 01 SlutionsDocument26 pages01 SlutionsAsif AhnafNo ratings yet
- Solutions GuideDocument5 pagesSolutions GuideREHAN --No ratings yet
- SH Hetler in PakDocument14 pagesSH Hetler in Pakpatiser mNo ratings yet
- CH 1 S Cbse BDocument14 pagesCH 1 S Cbse BIchigo KurosakiNo ratings yet
- Random Notes Class 12 ChemistryDocument87 pagesRandom Notes Class 12 Chemistryankitajamatia06No ratings yet
- Chemical Equation Show Reactants Combining in A Fixed Molar RatioDocument9 pagesChemical Equation Show Reactants Combining in A Fixed Molar RatioKristinaNo ratings yet
- CERTIFICATE ch1Document18 pagesCERTIFICATE ch1manvir681singhNo ratings yet
- 1.9 Colligative Properties of SolutionsDocument9 pages1.9 Colligative Properties of Solutionsfikerdereje697No ratings yet
- Learning Material (CHEMISTRY)Document141 pagesLearning Material (CHEMISTRY)aayanNo ratings yet
- Colligative Properties: 1. Vapor-Pressure Lowering 2. Boiling Point ElevationDocument30 pagesColligative Properties: 1. Vapor-Pressure Lowering 2. Boiling Point ElevationketantchaudhariNo ratings yet
- X-Ray Diffraction Document (1) (4) - 1Document9 pagesX-Ray Diffraction Document (1) (4) - 1MEEZAN TVNo ratings yet
- Solution in One PageDocument2 pagesSolution in One Pageraiprisha06No ratings yet
- Concentration of Solutions NotesDocument5 pagesConcentration of Solutions NotesIonacer ViperNo ratings yet
- New Basic Chemistry 2 Solution (Ideal Solution and Colligative PropertiesDocument83 pagesNew Basic Chemistry 2 Solution (Ideal Solution and Colligative PropertiesRetno Ayu PuspitaNo ratings yet
- Vdocuments - MX Colligative Property Made by Chinmay Jagadev PattanayakDocument18 pagesVdocuments - MX Colligative Property Made by Chinmay Jagadev PattanayakAbhishek NegiNo ratings yet
- Hukum RoultDocument25 pagesHukum RoultZaqiya zahwa alifaNo ratings yet
- Lecture 1Document31 pagesLecture 1Nimit RiniNo ratings yet
- Solubility of Solids in Liquids ExperimentDocument2 pagesSolubility of Solids in Liquids ExperimentIrene Refaat FouadNo ratings yet
- Solutions FinalDocument12 pagesSolutions FinalAman DeepNo ratings yet
- Colligative Properties: BY Dr. V.N. GowardipeDocument25 pagesColligative Properties: BY Dr. V.N. GowardipeSammed ShigalliNo ratings yet
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- A Novel Method To Produce PD Nanoparticle Ink For Ink-Jet Printing TechnologyDocument5 pagesA Novel Method To Produce PD Nanoparticle Ink For Ink-Jet Printing Technologytpjoshi1No ratings yet
- A Collection of Papers Presented at The 22nd ECIS ConferenceDocument2 pagesA Collection of Papers Presented at The 22nd ECIS Conferencetpjoshi1No ratings yet
- P N N P N + (1 - N) P P N + P - N P N - N + P P P + N (P - P)Document1 pageP N N P N + (1 - N) P P N + P - N P N - N + P P P + N (P - P)tpjoshi1No ratings yet
- Introduction & Basic Concepts of Thermodynamics: Reading ProblemsDocument6 pagesIntroduction & Basic Concepts of Thermodynamics: Reading Problemstpjoshi1No ratings yet
- ContentDocument1 pageContenttpjoshi1No ratings yet
- Applications of Raoult's LawDocument1 pageApplications of Raoult's Lawtpjoshi1No ratings yet
- The C Puzzle BookDocument93 pagesThe C Puzzle Bookabhijeetnayak67% (3)
- Band Gaps and Electronics Structure of PerovskitesDocument12 pagesBand Gaps and Electronics Structure of PerovskitesThanh Long TaNo ratings yet
- Chapter 1 - Steam GenerationDocument23 pagesChapter 1 - Steam GenerationAzhan FikriNo ratings yet
- Introduction To Research MethodsDocument11 pagesIntroduction To Research MethodsKamlakar SadavarteNo ratings yet
- IntegersDocument20 pagesIntegersMahobeNo ratings yet
- Adjectives 4Document34 pagesAdjectives 4Delia Bolasoc100% (1)
- Chapter 8Document7 pagesChapter 8Maiane JunqueiraNo ratings yet
- Application Note 31 Monitoring Quicklime Monitoring PDFDocument4 pagesApplication Note 31 Monitoring Quicklime Monitoring PDFomar rahmounNo ratings yet
- HashingDocument75 pagesHashingThz EsyyNo ratings yet
- Programming: Simon ScheideggerDocument90 pagesProgramming: Simon ScheideggerRuben KempterNo ratings yet
- Pseudocode Is A Technique Used To Describe The Distinct Steps of An Algorithm in ADocument3 pagesPseudocode Is A Technique Used To Describe The Distinct Steps of An Algorithm in AChristian Doson EstilloreNo ratings yet
- Adelio Lattuada TL10AV & TL10Document7 pagesAdelio Lattuada TL10AV & TL10yaser radNo ratings yet
- Universal Law of GravitationDocument17 pagesUniversal Law of GravitationScionNo ratings yet
- Analytic Geometry Parabola ProblemsDocument14 pagesAnalytic Geometry Parabola ProblemsOjit QuizonNo ratings yet
- What Is A Stress Intensification FactorDocument7 pagesWhat Is A Stress Intensification FactorMahendra RathoreNo ratings yet
- Caie As Computer Science 9618 Theory v3Document20 pagesCaie As Computer Science 9618 Theory v3James HoangNo ratings yet
- PDF Sesion de Aprendizaje de Comunicacion Leemos y Cantamos Canciones Criollas Lambayecanas - CompressDocument6 pagesPDF Sesion de Aprendizaje de Comunicacion Leemos y Cantamos Canciones Criollas Lambayecanas - CompressJulia Navarro CheroNo ratings yet
- DTC P1200 Fuel Pump Relay/ECU Circuit MalfunctionDocument4 pagesDTC P1200 Fuel Pump Relay/ECU Circuit MalfunctiononealNo ratings yet
- Simulation Tool ComparisonDocument8 pagesSimulation Tool ComparisonsmautifNo ratings yet
- GGGB6023 Tugasan Tutorial 3 - P69060 Mior SyazrilDocument5 pagesGGGB6023 Tugasan Tutorial 3 - P69060 Mior SyazrilAmizan AbdullahNo ratings yet
- Study of Educational Aspiration and Socio-Economic Status of Secondary School StudentsDocument11 pagesStudy of Educational Aspiration and Socio-Economic Status of Secondary School StudentsvivekNo ratings yet
- 4 Activity Guide and Evaluation Rubric - Unit 2 - Task 4 - Lets Talk and Share - Speaking Task - En.esDocument8 pages4 Activity Guide and Evaluation Rubric - Unit 2 - Task 4 - Lets Talk and Share - Speaking Task - En.esFabiana Cataño gomezNo ratings yet
- Summative Test in Grade 10 Science PDF FreeDocument2 pagesSummative Test in Grade 10 Science PDF FreeMalyn ReyesNo ratings yet
- LAB 7 - Getting Started With Google BigQueryDocument10 pagesLAB 7 - Getting Started With Google BigQueryRama VNo ratings yet
- DCS800 Firmware Manual EnglishDocument298 pagesDCS800 Firmware Manual EnglishMadson FernandesNo ratings yet
- High-Resolution Screening of Metabolite-Like Lead LibrariesDocument114 pagesHigh-Resolution Screening of Metabolite-Like Lead LibrariesBuscador AlfaNo ratings yet
- Mha Mca Cet SyllabusDocument20 pagesMha Mca Cet Syllabusm kumarNo ratings yet
- Shared Memory ArchitectureDocument2 pagesShared Memory ArchitectureNeethu RajeshNo ratings yet
- Evaluation of Yogurt Production Line Simulation Using Arena SoftwareDocument8 pagesEvaluation of Yogurt Production Line Simulation Using Arena Softwareg defNo ratings yet