Professional Documents
Culture Documents
Ionic Equilibrium Practice Problems
Uploaded by
Naganarasaiah GoudOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ionic Equilibrium Practice Problems
Uploaded by
Naganarasaiah GoudCopyright:
Available Formats
SR +LT BIPC PROG-1 DAY-8 PRACTICE ASSIGNMENT
SUBJECT: CHEMISTRY
CHAPTER: Chemical & Ionic equilibrium,
IONIC EQUILIBRIUM
1. The most important buffer in the blood consists of
(1) HCl and Cl (2) H 2 CO 3 and HCO 3 (3) H 2 CO 3 and Cl (4) HCl and HCO 3
Sol: (2) Blood consists of H 2 CO 3 HCO 3 buffer solution.
2. The solubility product of AgI at 25 C is 1. 0 10 16 mol 2 L2 . The solubility if AgI in 10 4 N solution of
KI at 25 C is approximately (in mol l 1 )
(1) 1 .0 10 8 (2) 1 . 0 10 16 (3) 1 . 0 10 12 (4) 1 . 0 10 10
Sol: (3) AgI ⇌ Ag I ; K sp S 2 10 4 S
(s ) (s )
1 .0 10 16 mol 2
S 4
1 10 12 2
10 l
M M
3. The pH of the solution: 5 mL of , HCl 10 mL of NaOH is
5 10
(1) 5 (2) 3 (3) 7 (4) 8
1
Sol: (3) Milliequivalents of HCl 5 1
5
1
Milliequivalents of NaOH 10 1
10
M M
5 ml HCl 10 ml HCl
5 5
Hence the solution will be neutral i.e., pH 7 .
4. Given that the dissociation constant for H 2O is K w 1 10 14 mole 2 / litre 2 . What is the pH of a 0.001 molar
KOH solution
(1) 10 11 (2) 10 3 (3) 3 (4) 11
Sol: (4) pH 14 pOH 14 3 11
5. The pH of 0.1 M solution of the following salts increases in the order
(1) NaCl NH 4 Cl NaCN HCl (2) HCl NH 4 Cl NaCl NaCN
(3) NaCN NH 4 Cl NaCl HCl (4) HCl NaCl NaCN NH 4 Cl
Sol: (2) HCl is strong acid. In its .1M solution, [H ] 0 .1 M and hence, pH 1
NH 4 Cl(aq ) hydrolyses in solution and give acidic solution which is less acidic than .1M HCl . NaCl is not
hydrolysed in aqueous solutions. Its pH 7 NaCN undergoes hydrolysis in solution to give alkaline
solution. So that pH increases in the order, HCl NH 4 Cl NaCl NaCN
NARAYANA MEDICAL ACADEMY 1
6. The degree of hydrolysis in hydrolytic equilibrum A H 2O ⇌ HA OH at salt concentration of 0.001
M is
K a 1 10 5
(1) 1 10 3 (2) 1 10 4 (3) 5 10 4 (4) 1 10 6
Kw 10 14
Sol: (1) K h 10 9
Ka 1 10 5
Kh 1 10 9
Kh 2C ; 1 10 3
C . 001
7. If pK b for fluoride ion at 25 o C is 10.83, the ionisation constant of hydrofluoric acid in water at this
temperature is
(1) 1. 74 10 3 (2) 3 .52 10 3 (3) 6. 75 10 4 (4) 5 . 38 10 2
Sol: (3) K a Kb Kw
Kw 10 14
K a 6. 75 10 4
Kb 1 .48 10 11
8. If the hydrogen ion concentration of a given solution is 5 .5 10 3 mol litre 1 , the pH of the solution will
be
(1) 2.26 (2) 3.40 (3) 3.75 (4) 2.76
3
Sol: (1) [H ] 5 .5 10 mole/litre
pH log [H ] ; pH log [5 . 5 10 3 ] ; pH 2 .26
[salt]
9. Henderson’s equation is pH pK a + log . If the acid gets half neutralized the value of pH will be :
[acid ]
[ pK a 4 .30 ]
(1) 4.3 (2) 2.15 (3) 8.60 (4) 7
[Salt]
Sol: (1) pH pK a log
[Acid]
1
pH 4 . 3 log 2 4 .3 log 1 ; pH 4 . 3 0 4 .3
1
2
10. The pH of a 0.01 M solution of acetic acid having degree of dissociation 12.5% is
(1) 5.623 (2) 2.903 (3) 3.723 (4) 4.509
1 .25
Sol: (2) [H ] C 0 . 01
100
H 1 .25 10 3 ; pH between 2 or 3 2.90
11. Which of the following solutions will have pH close to 1.0
(1) 100 ml of M HCl 100 ml of M NaOH (2) 55 ml of M HCl 45 ml of M NaOH
10 10 10 10
(3) 10 ml of M HCl 90 ml of M NaOH (4) 75 ml of M HCl 25 ml of M NaOH
10 10 5 5
1 1
Sol: (4) M.eq. of HCl 75 15 ; M.eq. of NaOH 25 5
5 5
Total No. of eq. 15 5 10
Total volume = 100
10 1
Normality , [H ] 10 1 M
100 10
NARAYANA MEDICAL ACADEMY 2
12. In which of the following solvents will AgBr have the highest solubility
(1) 10 3 M NaBr (2) 10 3 M NH 4 OH (3) Pure water (4) 10 3 M HBr
Sol: (2) AgBr are not dissolved in NaBr and HBr due to common ion effect. And pure water is a neutral
solvent. They do not have ions.
13. How many grams of CaC 2 O 4 will dissolve in distilled water to make one litre of saturated solution ?
(Solubility product of CaC 2 O 4 is 2 .5 10 9 mole 2 litre 2 and its molecular weight is 128)
(1) 0.0064 gm (2) 0.0128 gm (3) 0.0032 gm (4) 0.0640 gm
Sol: (1) CaC 2 O 4 is a binary electrolyte. Then solubility is
S K sp 2 . 5 10 9 5 10 5 mole/l. 0.0064 gm/l.
14. The solubility product of CuS , Ag 2 S , HgS are 10 31 , 10 44 , 10 54 respectively. The solubilities of these
sulphides are in the order
(1) Ag 2 S CuS HgS (2) Ag 2 S HgS Cus (3) HgS Ag 2 S Cus (4) CuS Ag 2 S HgS
Sol: (1) Ag 2 S CuS HgS
15. The solubility product constant K sp of Mg(OH )2 is 9. 0 10 12 . If a solution is 0 .010 M with respect to
Mg 2 ion, what is the maximum hydroxide ion concentration which could be present without causing
the precipitation of Mg (OH )2
(1) 1 . 5 10 7 M (2) 3 . 0 10 7 M (3) 1 . 5 10 5 M (4) 3 . 0 10 5 M
Sol: (4) Mg (OH ) 2 ⇌ Mg 2OH2
K sp S (2 S )
K sp S 4 S 2
K sp 9 10 12
S2 2 . 25 10 10
S 4 .010 4
S 2 .25 10 10 1 . 5 10 5 m/l
16. If the K b value in the hydrolysis reaction B H 2O ⇄ BOH H is 1 . 0 10 6 , then the hydrolysis
constant of the salt would be
(1) 1 . 0 10 6 (2) 1 . 0 10 7 (3) 1 . 0 10 8 (4) 1 . 0 10 9
Kw 10 14
Sol: (3) For hydrolysis of B ; K H 10 8 .
Kb 10 6
17. For a sparingly soluble salt A p Bq , the relationship of its solubility product (LS ) with its solubility (S ) is
(1) Ls S p q . p p .q q (2) Ls S p q . p q .q p (3) Ls S pq . p p .q q (4) L s S pq .( p .q )p q
Sol: (1) Ap Bq ⇌ pA 1 qB p
Ls [ A q ]p [ B p ]q ( p S )p (q S )q S p q . p p .q q .
18. Arrange NH 4 , H 2 O, H 3 O , HF and OH in increasing order of acidic nature
(1) H 3 O NH 4 HF OH H 2 O (2) NH 4 HF H 3 O H 2 O OH
(3) OH H 2 O NH 4 HF H 3 O (4) H 3 O HF H 2 O NH 4 OH
Sol: (3) H 3 O HF NH 4 H 2 O OH .Acidic nature is decreasing order.
19. How many grams of CaC 2O 4 (molecular weight = 128) on dissolving in distilled water will give a
saturated solution [K sp (CaC 2 O4 ) 2. 5 10 9 mol 2 l 2 ]
(1) 0.0064 g (2) 0.1280 g (3) 0.0128 g (4) 1.2800 g
Sol: (1) Solubility of CaC 2 O4 K sp 2. 5 10 9 5 10 5 molL1 5 10 5 128 640 10 5 0. 0064 g
NARAYANA MEDICAL ACADEMY 3
20. If the concentration of CrO 4 ions in a saturated solution of silver chromate is 2 10 4 . Solubility product
of silver chromate will be
(1) 4 10 8 (2) 8 10 12 (3) 12 10 12 (4) 32 10 12
Sol: (4) K sp of Ag 2 CrO 4 [ Ag ] 2 [Cro 4 ]
CrO 4 2 10 4 then Ag 2 2 10 4
K sp (4 10 4 ) 2 (2 10 4 ) 32 10 12
21. According to Bronsted-Lowry concept, the correct order of relative strength of bases follows the order
(1) CH 3 COO Cl OH (2) CH 3 COO OH Cl
(3) OH CH 3 COO Cl (4) OH Cl CH 3 COO
Sol: (3) Relative strength of bases can be shown by their conjugated acids.
Conjugate acid of OH is H 2O which is a weak acid conjugate acid of CH 3 COO is CH 3 COOH
which is stronger than H 2O . while conjugate acid of Cl is HCl which is strongest out of there. so the
order of relative strength of bases is OH CH 3 COO Cl .
22. H 2 SO 4 OH SO 42 H 2 O Which is correct about conjugate acid base pair
(1) HSO 42 is conjugate acid of base SO 42 (2) HSO 4 is conjugate base of acid SO 42
(3) SO 4 is conjugate acid of base HSO 4 (4) None of these
Sol: (1) HSO 4 OH SO 42 H 2 O
Conjugate acid Conjugate base
23. Which may be added to one litre of water to act as a buffer
(1) One mole of HC 2 H 3 O 2 and 0.5 mole of NaOH
(2) One mole of NH 4 Cl and one mole of HCl
(3) One mole of NH 4 OH and one mole of NaOH
(4) One mole of HC 2 H 3 O2 and one mole of HCl
Sol: (1) One mole oxalic acid & 0.5 mole of NaOH will make.
24. Which of the following base is weakest
(1) NH 4 OH : K b 1 . 6 10 6 (2) C 6 H 5 NH 2 : K b 3 . 8 10 10
(3) C 2 H 5 NH 2 : K b 5 .6 10 4 (4) C 6 H 7 N : K b 6 .3 10 10
Sol: (2) Smallest value of Kb indicates that aniline (C2 H 5 NH 2 ) is the weakest base.
25. HClO is a weak acid. The concentration of H ions in 0 . 1 M solution of HClO (K a 5 10 8 ) will be
equal to
(1) 7 . 07 10 5 m (2) 5 10 9 m (3) 5 10 7 m (4) 7 10 4 m
Sol: (1) [ H ] K a C 0.1 5 10 8 50 10 10 7.07 10 5 M
26. Upto what pH must a solution containing a precipitate of Cr(OH )3 be adjusted so that all of precipitate
dissolves
(When Cr 3 0 .1 mol / l, K sp 6 10 31 )
(1) Upto 4.4 (2) Upto 4.1 (3) Upto 4.2 (4) Upto 4.0
3 3
Sol: (4) K sp [Cr ][OH ]
6 10 31
[OH ] 3 K sp / Cr 3 6 10 30 ; [OH ] 1 .8 10 10
1 10 1
pOH (log 1 . 8 log 10 10 ) 10 0 .25 1 11 .25 ; pH 14 11 .25 2 .27
NARAYANA MEDICAL ACADEMY 4
27. NH 4 Cl is acidic, because
(1) On hydrolysis NH 4 Cl gives weak base NH 4 OH and strong acid HCl
(2) Nitrogen donates a pair of electron
(3) It is a salt of weak acid and strong base
(4) On hydrolysis NH 4 Cl gives strong base and weak acid
Sol: (1) N H 4C l H 2O N H 4O H H C l
NH 4 Cl is a salt of weak base & strong acid so solution will be acidic.
28. A solution of weak acid HA containing 0.01 moles of acid per litre of solutions has pH 4 . The
percentage degree of ionisation of the acid and the ionisation constant of acid are respectively
(1) 1 %, 10 6 (2) 0 .01 %, 10 4 (3) 1 %, 10 4 (4) 0 .01 %, 10 6
Sol: (1) H C
H 10 4
2 10 6
C 10
29. The pH of a buffer solution containg 0.2 mole per litre CH 3 COONa and 1.5 mole per litre CH 3 COOH is
(Ka for acetic acid is 1 . 8 10 5 )
(1) 4.87 (2) 5.8 (3) 2.4 (4) 9.2
[Salt] 0.2
Sol: (1) pH log K a log log [1 . 8 10 5 ] log 4 . 87
[Acid] 0 .1
30. 100 mL of 0 .04 N HCl aqueous solution is mixsed with 100 mL of 0.02 N NaOH solution. The pH of the
resulting solution is
(1) 1.0 (2) 1.7 (3) 2.0 (4) 2.3
Sol: (3) N1V1 . 04 100 4
N 2 V2 . 02 100 2
N 1 V1 N 2 V2 N 3 V3
4 2 N 3 200 , N 3 10 2 M
1 1
pH log 10 log 10 2.
H 10 2
31. An alcoholic drink substance pH 4 .7 then OH ion concentration of this solution is (K w 10 14 mol 2 / l 2 )
(1) 3 10 10 (2) 5 10 10 (3) 1 10 10 (4) 5 10 8
Sol: (2) pH 4 .7
pH pOH 14 ; pH 14 4. 7 ; pOH 9 .3
[OH ] Antilog [ pOH ] Antilog [9 .3 ]
[OH ] 5 10 10
32. In its 0.2 M solution, an acid ionises to an extent of 60%. Its hydrogen ion concentration is
(1) 0.6 M (2) 0.2 M (3) 0.12 M (4) None of these
Sol: (3) [H ] C . , 0 .2 0.60 0 .12 M
33. pH of 0.1 M NH 3 aqueous solution is (K b 1 .8 10 5 )
(1) 11.13 (2) 12.5 (3) 13.42 (4) 11.55
Sol: (1) NH 4 OH ⇌ NH 4 OH
1 .8 10 5
K b C 2 ; 2 ; 1 .34 10 3
.1
[OH ] . C 1 .34 10 3 .1
NARAYANA MEDICAL ACADEMY 5
1
pOH log 10 ; pOH 2. 87
1 .34 10 4
pH pOH 14 ; pH 2. 87 14
pH 14 2 . 87 ; pH 11 .13
34. 40 mg of pure sodium hydroxide is dissolved in 10 litres of distilled water. The pH of the solution is
(1) 9.0 (2) 10 (3) 11 (4) 12
Solute in 1 litre solution
Sol: (2) M
Molecular weight of solute
40 10 3 1
10 4 M
40 10
1 1
pOH log 10
log 10 4
[OH ] 10 4
pH pOH 14 ; pH 4 14 pH 10 .
35. Solubility of PbI 2 is 0.005 M. Then, the solubility product of PbI 2 is
(1) 6 .8 10 6 (2) 6 . 8 10 6 (3) 2 .2 10 9 (4) None of these
Sol: (4) PbI 2 Pb I 2
x 2x
K sp 4 x 3 4 (.005 )3 4 .005 .005 .4 10 6 .
36. A monoprotic acid in a 0. 1 M solution ionizes to 0.001%. Its ionisation constant is
(1) 1 . 0 10 3 (2) 1 . 0 10 6 (3) 1 . 0 10 8 (4) 1 . 0 10 11
Sol: (4) Monoprotic acid HA
HA ⇌ H A
Ionisation constant = ?
0 . 001
0 .001 % 10 5
100
2 [10 5 ] 2
K 10 11 .
V 10
37. Select the pK a value of the strongest acid from the following
(1) 1.0 (2) 3.0 (3) 2.0 (4) 4.5
Sol: (1) pKa then strongest acid
pKa then weak acid
1
pKa
Acidic strength
38. At 90°C, pure water has H 3O ion concentration of 10 6 mol / L1 . The K w at 90°C is
(1) 10 6 (2) 10 14 (3) 10 12 (4) 10 8
Sol: (3) H 3 O H 2 O H 6
10 6 10 6 10
K w [ H 2 O ][ H ] = [10 6 ][10 6 ] 10 12
39. By adding 20 ml 0 .1 N HCl to 20 ml 0 .1 N KOH , the pH of the obtained solution will be
(1) 0 (2) 7 (3) 2 (4) 5
Sol: (2) Neutralization reaction will takes place and form salt of strong acid and strong base. Which does not
hydrolysed and thus pH 7 .
NARAYANA MEDICAL ACADEMY 6
CHEMICAL EQUILIBRIUM
40. One mole of SO 3 was placed in a litre reaction vessel at a certain temperature. The following
equilibrium was established 2SO 3 ⇌ 2 SO 2 O 2 At equilibrium 0.6 moles of SO 2 were formed. The
equilibrium constant of the reaction will be
(1) 0.36 (2) 0.45 (3) 0.54 (4) 0.675
Sol: (4) 2 SO 3 ⇌ 2 SO 2 O 2
(1 0.6 ) (0 .6 ) ( 0 . 3)
2
[SO 2 ] [O 2 ] 0. 6 0. 6 0. 3
Kc 0. 675 .
[SO 3 ] 0. 4 0. 4
41. For the following homogeneous gas reaction 4 NH 3 5 O 2 ⇌ 4 NO 6 H 2 O , the equilibrium constant K c
has the dimension of
(1) Conc 10 (2) Conc 1 (3) Conc 1 (4) It is dimensionless
n
Sol: (2) K has the units of (conc. ) , where n 10 9 1
42. Consider the imaginary equilibrium 4A + 5B ⇌ 4X + 6Y. The equilibrium constant K c has the unit
(1) Mole2 litre-2 (2) Litre mole-1 (3) Mole litre-1 (4) Litre2 mole-2
Sol: (3) Unit of K c (unit of concentration) = (mole litre–1)n
n
n = 10 – 9 = 1
K c mol Litre–1.
43. For the reaction CO (g) 2 H 2 (g) ⇌ CH 3 OH (g) , true condition is
(1) K p Kc (2) K p K c (3) K p Kc (4) Kc 0 but K p 0
Sol: (3) When nr n p then K p K c
where nr = no. of moles of reactant
np = no. of moles of product.
1 Kp
44. For the reaction CO (g) O 2 (g) ⇌ CO 2 (g) ; is equivalent to
2 Kc
1
(1) 1 (2) RT (3) (4) (RT )1 / 2
RT
Sol: (3) For CO 1 O 2 ⇌ CO 2
2
1 1
1 1 Kp 1
K p Kc (RT ) 2 K c (RT ) 2 ;
Kc RT
45. 2 N 2 O 5 4 NO 2 O 2 what is the ratio of the rate of decomposition of N 2 O 5 to rate of formation of NO 2
(g) (g) (g )
(1) 1:2 (2) 2 : 1 (3) 1:4 (4) 4 :1
Sol: (2) 2 N 2 O5 HNO 2 O 2
1 K[ N 2 O 5 ]
Rate of decomposition of N 2 O5 .
2 dt
1 d[ NO 2 ]
Rate of formation of NO 2 .
4 dt
Ratio = 2 : 1
[ NH 3 ]2
46. The reaction quotient (Q) for the reaction N 2( g ) 3 H 2(g ) ⇌ 2 NH 3( g ) is given by Q . The reaction
[ N 2 ][ H 2 ]3
will proceed from right to left is (Where K c is the equilibrium constant)
(1) Q=0 (2) Q = K c (3) Q < K c (4) Q > Kc
Sol: (4) If Q K c reaction will proceed right to left to decrease concentration of product
NARAYANA MEDICAL ACADEMY 7
47. In the thermal dissociation of PCl 5 , the partial pressure in the gaseous equilibrium mixture is 1.0
atmosphere when half of PCl 5 is found to dissociate. The equilibrium constant of the reaction (K p ) in
atmosphere is
(1) 0.25 (2) 0.50 (3) 1.00 (4) 0.3
Sol: (4) PCl 5 ⇌ PCl 3 Cl 2
Initial conc. 1 0 0
At equilibrium 0.5 0.5 0.5
Px 2 1 0.5 0.5 0.5 0.5 1
Kp 0.3
(1 x 2 ) [1 (0 . 5 )2 ] 0 .75 3
48. HI was heated in a closed tube at 440 o C till equilibrium is obtained. At this temperature 22% of HI was
dissociated. The equilibrium constant for this dissociation will be
(1) 0.282 (2) 0.0796 (3) 0.0199 (4) 1.99
Sol: (3) 2HI ⇌ H 2 I2
Initial conc. 2 moles 0 0
at equilibrium 22 2 0.22 0.22
100
2 0 . 44 1 .56
[H 2 ] [ I2 ] 0 .22 0 . 22
K 0 . 0199 .
[HI ]2 [1 . 56 ]2
49. The following equilibrium exists in aqueous solution CH 3 COOH ⇌ CH 3 COO H . If dilute HCl is
added without a change in temperature, then the
(1) Concentration of CH 3 COO will increase (2) Concentration of CH 3 COO will decrease
(3) Equilibrium constant will increase (4) Equilibrium constant will decrease
Sol: (2) When adding HCl in CH 3 COOH solution the concentration of H is increased. So reaction is
proceed in reverse direction and the concentration of CH 3 COO is decreased.
50. Which of the following is not favourable for SO 3 formation 2 SO 2 (g) O 2 (g) ⇌ 2 SO 3 (g); H 45 . 0 kcal
(1) High pressure (2) High temperature
(3) Decreasing SO 3 concentration (4) Increasing reactant concentration
Sol: (2) The reaction is exothermic so high temperature will favour backward reaction.
51. 46 gm of nitrogen are present in 5 litre solution, the active mass of nitrogen is
(1) 0.2 (2) 0.06 (3) 0.4 (4) 0.08
wt.in gm /molecular wt. 46 28 2
Sol: (3) Active mass moles 0.4
litre V in litre 5 5
52. For the system 2 A(g) B(g) ⇌ 3 C(g) , the expression for equilibrium constant K is
[ 2 A] [ B] [ A] 2 [ B] [3 C ] [C ]3
(1) (2) (3) (4)
[3 C ] [C ]3 [ 2 A] [ B] [ A] 2 [ B]
[C ]3
Sol: (4) K .
[ A]2 [ B]
53. If concentration of reactants is increased by ' x ' , then K becomes
(1) ln ( K / x ) (2) K / x (3) Kx (4) K
Sol: (4) There is no effect of change in concentration on equilibrium constant
NARAYANA MEDICAL ACADEMY 8
You might also like
- Lehninger Principles of Biochemistry 6th Edition Nelson Solutions ManualDocument24 pagesLehninger Principles of Biochemistry 6th Edition Nelson Solutions ManualHollyBurnsPhDwadn100% (35)
- 17PS2ADocument4 pages17PS2ASeamus AlaricNo ratings yet
- CH 2 - ProblemsDocument6 pagesCH 2 - ProblemsKhris Griffis94% (17)
- Process CheckLists P&ID Rev 3Document3 pagesProcess CheckLists P&ID Rev 3Farhan AhmedNo ratings yet
- Ionic Equilibrium-03-Objective and Subjective Assignments and Answer SheetDocument16 pagesIonic Equilibrium-03-Objective and Subjective Assignments and Answer SheetRaju SinghNo ratings yet
- Ionic Equilibrium DPP Nitesh DevnaniDocument16 pagesIonic Equilibrium DPP Nitesh DevnaniYUKTESH YuBoNo ratings yet
- Ionic EquilibriumDocument55 pagesIonic Equilibriumharshul jainNo ratings yet
- Quiz-Ionic Equilibrium-Vd - SNDDocument4 pagesQuiz-Ionic Equilibrium-Vd - SNDObama binladenNo ratings yet
- 03 - 9TH Co-Iit - P-B - Chemistry - Ionic Equilibrium AssignmentDocument6 pages03 - 9TH Co-Iit - P-B - Chemistry - Ionic Equilibrium AssignmentramkarthikeyareddyNo ratings yet
- 04ionic Equilibrium Set Test Final EDocument5 pages04ionic Equilibrium Set Test Final EBad boy boyNo ratings yet
- Ans-Sol JEEMain-2022 Phase-2 25-07-2022 M Chemistry FINALDocument7 pagesAns-Sol JEEMain-2022 Phase-2 25-07-2022 M Chemistry FINALChirayu SharmaNo ratings yet
- Jee Main 2017 Test Paper Code - C Questions With SolutionsDocument33 pagesJee Main 2017 Test Paper Code - C Questions With SolutionsAneesh ChawlaNo ratings yet
- Ionic QuestionsDocument4 pagesIonic QuestionsSubharna ChauhanNo ratings yet
- Ionic Equilibrium Practice SheetDocument2 pagesIonic Equilibrium Practice SheetRSLNo ratings yet
- Assignment 1Document5 pagesAssignment 1Leo PalNo ratings yet
- 8 Ionic Equilibrium MCQsDocument8 pages8 Ionic Equilibrium MCQsANIKET BATTINWARNo ratings yet
- Get Equipped Jee Advanced and ComprehensionDocument23 pagesGet Equipped Jee Advanced and ComprehensionAjay SinghNo ratings yet
- Ionic Equilibrium: DPP 04 (Of Lec 08) - Arjuna JEE 2024Document2 pagesIonic Equilibrium: DPP 04 (Of Lec 08) - Arjuna JEE 2024Manas DubeyNo ratings yet
- Ionic EquilibriumDocument2 pagesIonic EquilibriumVidhuPandey100% (1)
- 06.acids and Bases 93-116Document6 pages06.acids and Bases 93-116eamcetmaterialsNo ratings yet
- Ionic Equilibrium Practice QuestionsTITLE Ionic Equilibrium Explanations TITLE Ionic Equilibrium Practice ProblemsDocument3 pagesIonic Equilibrium Practice QuestionsTITLE Ionic Equilibrium Explanations TITLE Ionic Equilibrium Practice ProblemsMuffadal AlaviNo ratings yet
- Mole Concept _ Practice Sheet 01 __ Lakshya 11th NEET Rapid Revision CourseDocument7 pagesMole Concept _ Practice Sheet 01 __ Lakshya 11th NEET Rapid Revision CoursemehranantipodalsNo ratings yet
- EquilibriumDocument22 pagesEquilibriumEzhil MukilNo ratings yet
- Acids BasesDocument34 pagesAcids BasesPrasad YarraNo ratings yet
- Intensive Revision Program of Physical Chemistry: By: Brijesh Jindal SirDocument9 pagesIntensive Revision Program of Physical Chemistry: By: Brijesh Jindal SirHudsun HornetNo ratings yet
- Acids and Bases Key ConceptsDocument6 pagesAcids and Bases Key ConceptsKrishna TyagiNo ratings yet
- Acids, Bases & Salt (Assignment - 2)Document4 pagesAcids, Bases & Salt (Assignment - 2)Garv100% (1)
- Exam 3 302-SolutionsDocument9 pagesExam 3 302-Solutionshuyentran1212No ratings yet
- Ques68 176abe2mcDocument14 pagesQues68 176abe2mcKerimberdiNo ratings yet
- Understanding Salt HydrolysisDocument7 pagesUnderstanding Salt HydrolysisKamlesh YadavNo ratings yet
- 9F. CH Nh2 (0.12 Mole, PK, 3.3) Is Added To 0.08 Moles of HCL and The Solution Is Diluted To SolutionDocument14 pages9F. CH Nh2 (0.12 Mole, PK, 3.3) Is Added To 0.08 Moles of HCL and The Solution Is Diluted To SolutionSandipan SamantaNo ratings yet
- 7.0 Ionic Equilibria: TutorialDocument13 pages7.0 Ionic Equilibria: Tutorializatirfan00No ratings yet
- Lehninger Principles of Biochemistry 6th Edition Nelson Solutions ManualDocument36 pagesLehninger Principles of Biochemistry 6th Edition Nelson Solutions Manualquakingeloign.ij6fg100% (41)
- Ionic Equilibrium Problems SolvedDocument2 pagesIonic Equilibrium Problems SolvedNinad Puranik0% (1)
- Chemistry EquilibriumDocument31 pagesChemistry EquilibriumAbhinavNo ratings yet
- Acid Base CH 16 ComprehensiveDocument4 pagesAcid Base CH 16 ComprehensiveAidah AmirNo ratings yet
- Chem 126 Common 2 Spring 2014 ABSWERS 23 Corrected IIDocument7 pagesChem 126 Common 2 Spring 2014 ABSWERS 23 Corrected IIjnv jnecionwNo ratings yet
- Ionic Equilibrium: (Physical Chemistry)Document8 pagesIonic Equilibrium: (Physical Chemistry)MAHI POPLINo ratings yet
- Ionic Equilibrium Objective Type QuestionsDocument22 pagesIonic Equilibrium Objective Type QuestionskeshavNo ratings yet
- Ionic Equlibrium QuestionsDocument19 pagesIonic Equlibrium Questionskishangopi123No ratings yet
- Modern Approach to Chemical Calculations: A Concise SEO-Optimized TitleDocument13 pagesModern Approach to Chemical Calculations: A Concise SEO-Optimized TitleAman9692No ratings yet
- Chemistry CPP Cat-3Document18 pagesChemistry CPP Cat-3faraazahmed70058No ratings yet
- Solution Manual For Lehninger Principles of Biochemistry Seventh EditionDocument27 pagesSolution Manual For Lehninger Principles of Biochemistry Seventh Editiontophet.layl0h8q100% (38)
- ChemistryDocument4 pagesChemistrySanath SaragadamNo ratings yet
- Ionic McqsDocument3 pagesIonic McqsMark AntonioNo ratings yet
- Jee 2014 Booklet3 HWT Ionic EquilibriumDocument10 pagesJee 2014 Booklet3 HWT Ionic EquilibriumvarunkohliinNo ratings yet
- Ionic Equilibrium CPP-1Document2 pagesIonic Equilibrium CPP-1phuliaikshuNo ratings yet
- BoookkkDocument24 pagesBoookkkayushy guptaNo ratings yet
- Sheet ST-8Document2 pagesSheet ST-8Sauri ChaitanyaNo ratings yet
- Ionic Equilibrium 520Document19 pagesIonic Equilibrium 520sarbajit mazumdarNo ratings yet
- Eamcet Part Test - 5Document6 pagesEamcet Part Test - 5udaysrinivasNo ratings yet
- 1 Acides Exercices SolutionsDocument4 pages1 Acides Exercices SolutionsNajib NouisserNo ratings yet
- chapter 14Document8 pageschapter 14dr.ibrahimsalemvpNo ratings yet
- Solubility calculationsDocument11 pagesSolubility calculationsLumir BobekNo ratings yet
- Ie +ceDocument2 pagesIe +ceVishnu kantNo ratings yet
- ACA-13 Physical ChemistryDocument30 pagesACA-13 Physical ChemistryAnonymous tricksNo ratings yet
- Chemistry Final Step-C - Mole ConceptDocument7 pagesChemistry Final Step-C - Mole ConceptAnas KhalidNo ratings yet
- Gen Chem II Exam 4 Titration, KSP Practice Problems f08Document5 pagesGen Chem II Exam 4 Titration, KSP Practice Problems f08Diego Marcelo Aragon CaqueoNo ratings yet
- Acid BaseDocument11 pagesAcid BaseSchmallNo ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- U-I 1.small Signal Analysis of Junction TransistorDocument22 pagesU-I 1.small Signal Analysis of Junction TransistorNaganarasaiah GoudNo ratings yet
- Timers and Counters in 8051 MicrocontrollersDocument5 pagesTimers and Counters in 8051 MicrocontrollersNaganarasaiah GoudNo ratings yet
- I/O port structure of the 8051 microcontrollerDocument40 pagesI/O port structure of the 8051 microcontrollerNaganarasaiah GoudNo ratings yet
- 4.day 6 DPT Chemistry PDFDocument5 pages4.day 6 DPT Chemistry PDFNaganarasaiah GoudNo ratings yet
- EC Unit 1 - Analysis of CE, CC, CB Circuits Using H ParametersDocument25 pagesEC Unit 1 - Analysis of CE, CC, CB Circuits Using H ParametersNaganarasaiah GoudNo ratings yet
- Multirate Digital Signal ProcessingDocument64 pagesMultirate Digital Signal Processingkishore_k777No ratings yet
- U-I 3.analysis of Transistor Using Simplified CircuitDocument6 pagesU-I 3.analysis of Transistor Using Simplified CircuitNaganarasaiah GoudNo ratings yet
- U-I 2.procedure For Analysis and Design ExamplesDocument10 pagesU-I 2.procedure For Analysis and Design ExamplesNaganarasaiah GoudNo ratings yet
- 8051 Timer/counter: Hsabaghianb at Kashanu - Ac.ir 1Document109 pages8051 Timer/counter: Hsabaghianb at Kashanu - Ac.ir 1amolmule123No ratings yet
- Multirate Digital Signal ProcessingDocument64 pagesMultirate Digital Signal Processingkishore_k777No ratings yet
- 5 Instruction SetDocument42 pages5 Instruction SetAnonymous PPCC2DAVNo ratings yet
- DSP-U5 - SC, AtoD, DtoADocument7 pagesDSP-U5 - SC, AtoD, DtoANaganarasaiah GoudNo ratings yet
- Programmable Interval Timer Initialization and OperationDocument19 pagesProgrammable Interval Timer Initialization and OperationNaganarasaiah GoudNo ratings yet
- WelcomeDocument35 pagesWelcomeNaganarasaiah GoudNo ratings yet
- DSP-U5 - SC, AtoD, DtoADocument7 pagesDSP-U5 - SC, AtoD, DtoANaganarasaiah GoudNo ratings yet
- DSP - Unit 4 - DecimationDocument14 pagesDSP - Unit 4 - DecimationNaganarasaiah GoudNo ratings yet
- Musical Sound Processing TechniquesDocument13 pagesMusical Sound Processing TechniquesNaganarasaiah GoudNo ratings yet
- Dma 8237Document12 pagesDma 8237Naganarasaiah GoudNo ratings yet
- Decimation 1Document9 pagesDecimation 1Naganarasaiah GoudNo ratings yet
- Decimation 1Document9 pagesDecimation 1Naganarasaiah GoudNo ratings yet
- Programmable Interval Timer 8253 (PIT) : COE305 LabDocument10 pagesProgrammable Interval Timer 8253 (PIT) : COE305 LabudayNo ratings yet
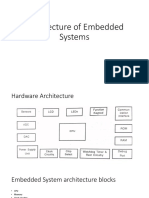
- Architecture of Embedded HardwareDocument35 pagesArchitecture of Embedded HardwareNaganarasaiah GoudNo ratings yet
- Piping Minimum Required Wall ThicknessDocument8 pagesPiping Minimum Required Wall ThicknessFadzil YahyaNo ratings yet
- navod-WH2310A EDocument34 pagesnavod-WH2310A EPeter FilloNo ratings yet
- MSDS GlukoseDocument5 pagesMSDS GlukosejuwaisrgNo ratings yet
- Resume Book Kelompok I English BiologyDocument5 pagesResume Book Kelompok I English Biologyuti nstNo ratings yet
- ABIR ProfileDocument30 pagesABIR ProfileMohamad NasserNo ratings yet
- MSDS LyseDocument14 pagesMSDS LyseidaNo ratings yet
- Meteorology - An Introduction To The Wonders of The WeatherDocument58 pagesMeteorology - An Introduction To The Wonders of The WeatherPreethi GopalanNo ratings yet
- Amana Gph13 Service Manual InstallationDocument43 pagesAmana Gph13 Service Manual Installationmazda8616No ratings yet
- Manual Shock Dose Into Seawater, Service and Fire Water PumpDocument12 pagesManual Shock Dose Into Seawater, Service and Fire Water PumpMohd HisammudinNo ratings yet
- CRB Handout 09.10.18Document19 pagesCRB Handout 09.10.18A.K.SINGHNo ratings yet
- Cruciferous VegetablesDocument13 pagesCruciferous Vegetablesapi-271257230No ratings yet
- Alcohols, Phenols and Ethers Test SeriesDocument5 pagesAlcohols, Phenols and Ethers Test SeriesCR foreverNo ratings yet
- Pananaliksik Sa Wikang FilipinoDocument21 pagesPananaliksik Sa Wikang FilipinoAbcedef Wyn Grey AbrasadoNo ratings yet
- Tire Chipping & ChunkingDocument7 pagesTire Chipping & ChunkingavdeoreNo ratings yet
- GTAW WPS for ASTM A240 TP 304LDocument1 pageGTAW WPS for ASTM A240 TP 304LAnand MakasanaNo ratings yet
- Cerasmart Cerasmart Cerasmart Cerasmart Cerasmart Cerasmart: UniversalDocument20 pagesCerasmart Cerasmart Cerasmart Cerasmart Cerasmart Cerasmart: Universalสุวิทย์ สะกิดตลิ่งNo ratings yet
- Forging & Die Design Course Optimizes ProcessesDocument5 pagesForging & Die Design Course Optimizes ProcessesSundar KaruppiahNo ratings yet
- Aerospace Structural Metals HandbookDocument74 pagesAerospace Structural Metals HandbookBrian Pinto50% (2)
- Hot Mix Asphalt Plant OperationsDocument81 pagesHot Mix Asphalt Plant OperationsJaikishan Kumaraswamy100% (3)
- Moisture Content of CoalDocument4 pagesMoisture Content of CoalSaad Ahmed100% (1)
- 090768.232 3.00 018 A e - ADocument111 pages090768.232 3.00 018 A e - ANoman Abu-FarhaNo ratings yet
- 1.4 Water Conservation: Water Monitoring and ManagementDocument3 pages1.4 Water Conservation: Water Monitoring and ManagementGaurav DuttaNo ratings yet
- Coordination and Organometallic ChemistryDocument42 pagesCoordination and Organometallic Chemistryfrank samndomiNo ratings yet
- FormualtionHandbook 12-2007Document0 pagesFormualtionHandbook 12-2007saidvaretNo ratings yet
- Oxidation of Thiols To Dissulfides With BromineDocument18 pagesOxidation of Thiols To Dissulfides With Brominejmario666No ratings yet
- Critical Review On The Analytical Methods For The Determination of Sulfur and Trace Elements in Crude OilDocument20 pagesCritical Review On The Analytical Methods For The Determination of Sulfur and Trace Elements in Crude OilLindaNo ratings yet
- Hydro EfbDocument7 pagesHydro EfbFA MonsterNo ratings yet
- Dupont Zytel Htn51g35hsl Nc01Document6 pagesDupont Zytel Htn51g35hsl Nc01josebernal_mzaNo ratings yet
- Hychill Refrigerants 1075 SdsDocument5 pagesHychill Refrigerants 1075 SdsLukasz PanfilNo ratings yet