Professional Documents
Culture Documents
Lab Assignment 2 Question
Uploaded by
anissfarhanaaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab Assignment 2 Question
Uploaded by
anissfarhanaaCopyright:
Available Formats
ANALYTICAL AND ORGANIC CHEMISTRY
CLD 10803
SEMESTER JANUARY 2020
CLD 10803 – Lab Assignment 2 (8% of final grade)
EXPERIMENT 3 AND EXPERIMENT 4
All the components in this assignment need to submit on 7/5/2020.
____/100 marks
Background and Content:
The purpose of this assignment is to help you to understand the methods of Experiment 3 and
Experiment 4 through the videos related to the experiments. In addition, it also help you to explore
connections between the lecture class learning and the experiments. In order to accomplish these
goals, this assignment has 2 sections.
Section 1 – Experiment 3 (60 marks)
In Section 1, there are 3 parts to fulfil this assignment.
Part 1 – Preparation of Ethyl Ethanoate (Ester) – VIDEO 1 (20 marks)
This part contains the specific questions to review the methodology, materials and
etc. of the preparation of ethyl ethanoate.
Part 2 – Utilization of FTIR for sample analysis – VIDEO 2 (20 marks)
This part to assist your understanding on how to prepare the samples and conduct
the analysis using FTIR.
Part 3 – Utilization of GC for sample analysis – VIDEO 3 (20 marks)
This part to help you to learn on how to analyse the samples using UV-vis.
Section 2 – Experiment 4 (40 marks)
In Section 2, there is 2 part in this assignment.
Part 1 – Preparation the sample solutions and standard solutions (20 marks)
This part to ensure that you know how to calculate the concentration in order to
prepare the standard solutions and plotting the calibration curve.
Part 2 – Analysis samples and standard solutions using HPLC – VIDEO 4 (20 marks)
This part to help you to understand the function of HPLC in the analysis.
Learning Outcomes:
The learning outcome (CLO) is CLO2 and will assess your cognitive domain on top of this CLO.
Grading:
The marks have been attributed to each parts.
Assignment submission:
All complete assignment need to submit through my email: nadiah_usm@yahoo.com with Microsoft
words or PDF format.
Other things to note:
Kindly put or paste your name, ID number and signature (e-signature) in your assignment documents.
This is individual assignment.
UNIVERSITI KUALA LUMPUR
(UNIKL MICET)
NNMY
ANALYTICAL AND ORGANIC CHEMISTRY
CLD 10803
SEMESTER JANUARY 2020
Section 1 – Experiment 3 (60 marks)
Part 1 – Preparation of Ethyl Ethanoate (Ester) – VIDEO 1 (20 marks)
1. Suggest the precaution that we need to take before this experiment started.
(4 marks)
2. During this experiment, she put the round bottom flask in ice bath before adding the
concentrated of sulfuric acid, 98%. Give the reason.
(4 marks)
3. Give the differences between reflux and distillation process.
(6 marks)
4. Give the function of sodium carbonate (Na2CO3) and calcium chloride (CaCl) in this
experiment.
(4 marks)
Part 2 – Utilization of FTIR for sample analysis – VIDEO 2 (20 marks)
1. Give full name of ATR.
(2 marks)
2. In this video, it shows the FTIR instrumentation diagram. Summarize clearly the process inside
FTIR equipment by using the same diagram in this video. You may give your answer in points.
(10 marks)
3. In FTIR spectrum, what is the information that you can obtain?
(4 marks)
4. By using the samples produced in Part 1 which is ethyl ethanoate, suggest the possible
functional group in this compound.
(4 marks)
Part 3 – Utilization of UV-vis for sample analysis – VIDEO 3 (20 marks)
1. Give the comparison between stationary phase and mobile phase.
(8 marks)
2. Determine the type of gas used for this instrument.
(2 marks)
3. Draw the GC instrument diagram in order to understand the process in the instrument.
(10 marks)
UNIVERSITI KUALA LUMPUR
(UNIKL MICET)
NNMY
ANALYTICAL AND ORGANIC CHEMISTRY
CLD 10803
SEMESTER JANUARY 2020
Section 2 – Experiment 4 (40 marks)
Part 1 – Preparation the sample solutions and standard solutions (20 marks)
In this part, you may refer any sources to answer the questions.
1. Show the calculation (with correct equation) of concentration of caffeine standards which are
2 M, 4 M, 6 M, 8 M and 10 M from caffeine stock solution which the concentration is 100 M.
You need to put all the answer after calculation in one table in Excel sheet.
E.g. (All calculation data need to put in table in Excel sheet)
Concentration Volume (after calculation)
2M
4M
6M
8M
10 M
(10 marks)
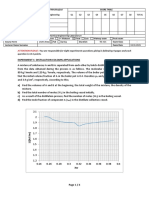
2. After analysis of caffeine standard solution and sample using HPLC instrument, the data can
be summarized as shown in Table 1. Plot the calibration curve (concentration (x-axis) vs area
(y-axis)) of caffeine standard solutions and determine the concentration of sample if the area
under the graph is 113698.
In the calibration curve, you need to:
a) Copy and paste the data in Table 1 in excel sheet.
b) Plot the graph by choosing SCATTER (points)
c) After the graph have been plot, right click and go to FORMAT TREADLINE, click set intercept,
display equation and display R2 value.
d) Then you can determine the concentration of sample.
Table 1: HPLC data for caffeine standard solutions
Concentration Area
2M 20356
4M 45638
6M 80123
8M 108963
10 M 124957
(10 marks)
UNIVERSITI KUALA LUMPUR
(UNIKL MICET)
NNMY
ANALYTICAL AND ORGANIC CHEMISTRY
CLD 10803
SEMESTER JANUARY 2020
Part 2 – Analysis samples and standard solutions using HPLC – VIDEO 4 (20 marks)
1. State the volume of sample needed to analyse using HPLC instrument.
(2 marks)
2. Draw the HPLC diagram in order to understand the process in the instrument.
(10 marks)
3. There is one technique she use to avoid ‘something’ in syringe before her injecting the samples
or standard solutions in HPLC instrument. Identify what is ‘something’ refer to and state the
technique she used.
(8 marks)
ALL THE BEST
UNIVERSITI KUALA LUMPUR
(UNIKL MICET)
NNMY
You might also like
- GARCH Models: Structure, Statistical Inference and Financial ApplicationsFrom EverandGARCH Models: Structure, Statistical Inference and Financial ApplicationsRating: 5 out of 5 stars5/5 (1)
- MET312 Non Destructive Testing SyllabusDocument8 pagesMET312 Non Destructive Testing SyllabusHARI KRISHNANNo ratings yet
- Non Destructive TestiDocument8 pagesNon Destructive TestiBen JoeNo ratings yet
- Met 312 NDTDocument8 pagesMet 312 NDTJanit JijiNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Final Theory Exam-307 June2012Document13 pagesFinal Theory Exam-307 June2012Jagadeesh EllilNo ratings yet
- M.Tech. (Computer Aided Chemical Engineering) (Effective From The Admitted Batch of 2019-20)Document35 pagesM.Tech. (Computer Aided Chemical Engineering) (Effective From The Admitted Batch of 2019-20)Gowri ShankarNo ratings yet
- Y1S2 Past PapersDocument45 pagesY1S2 Past PaperswaireriannNo ratings yet
- CHM580Document7 pagesCHM580Azreen AnisNo ratings yet
- Final Theory Exam-307 Dec11-FinalDocument14 pagesFinal Theory Exam-307 Dec11-FinalJagadeesh EllilNo ratings yet
- WS2019 2020Document6 pagesWS2019 2020ayisha.maharramovaNo ratings yet
- Vibration MeterDocument11 pagesVibration MeterNatNo ratings yet
- Using FTIR-ATR Spectroscopy To Teach The Internal Standard MethodDocument3 pagesUsing FTIR-ATR Spectroscopy To Teach The Internal Standard MethodEdgar VegaNo ratings yet
- M.tech - Manufacturing - and - Automation 2nd Year SllybusDocument7 pagesM.tech - Manufacturing - and - Automation 2nd Year SllybusShubham JangraNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Uj 39267+SOURCE1+SOURCE1.1Document7 pagesUj 39267+SOURCE1+SOURCE1.1Lilo KuleNo ratings yet
- Course Course Code Examination Time Spectrochemical Methods of Analysis CHM580 JUNE 2012 2 HoursDocument9 pagesCourse Course Code Examination Time Spectrochemical Methods of Analysis CHM580 JUNE 2012 2 HoursNur CichimaNo ratings yet
- Lab Sheet PDFDocument9 pagesLab Sheet PDFtehpohkeeNo ratings yet
- Semester - 3: Chemical EngineeringDocument135 pagesSemester - 3: Chemical EngineeringKevinNo ratings yet
- TLC Lab-Summer 2021 1Document2 pagesTLC Lab-Summer 2021 1Aidan Kyle SanglayNo ratings yet
- Proficiency Test: Charpy Impact Test (CIT 2004)Document39 pagesProficiency Test: Charpy Impact Test (CIT 2004)MehdiNo ratings yet
- Omed0104 2022 MayDocument4 pagesOmed0104 2022 MayIffa NooramNo ratings yet
- D C, P, C M E U E T, L (N C) : Important Instructions For StudentsDocument3 pagesD C, P, C M E U E T, L (N C) : Important Instructions For Studentsumair saleemNo ratings yet
- CE3001 Questions 2021-22Document5 pagesCE3001 Questions 2021-22suhel ahmadNo ratings yet
- Aplac m022 Final ReportDocument31 pagesAplac m022 Final ReportDoli MotorNo ratings yet
- 1 Dipl. Brew. Module 1: Unit 1.11 - Quality - Section 1.11.3Document14 pages1 Dipl. Brew. Module 1: Unit 1.11 - Quality - Section 1.11.3RiyanNo ratings yet
- Oriental Journal of Chemistry 28 (3) 1091-1098Document8 pagesOriental Journal of Chemistry 28 (3) 1091-1098Noureddine BarkaNo ratings yet
- Labsheet Translation Mechanical SystemDocument33 pagesLabsheet Translation Mechanical SystemMuhammad Ehsan Abdul Halim0% (1)
- Laboratory Report Submission FormDocument11 pagesLaboratory Report Submission FormDaniel IsmailNo ratings yet
- 2010 Oct QMT500Document8 pages2010 Oct QMT500Nurul Hidayah IbrahimNo ratings yet
- 2nd Year Scheme Syllabus AEDocument60 pages2nd Year Scheme Syllabus AEHareesha N GNo ratings yet
- Osha Id 125gDocument43 pagesOsha Id 125gZenal AbidinNo ratings yet
- Report Contribution Form: Che Laboratory CompositionDocument18 pagesReport Contribution Form: Che Laboratory CompositionEthan MuddNo ratings yet
- Aplac - t052 - Tensile Test For Metallic MaterialsDocument40 pagesAplac - t052 - Tensile Test For Metallic MaterialsSonja KostićNo ratings yet
- Osha Id 125gDocument43 pagesOsha Id 125gapriliandiniNo ratings yet
- Analytical Chemistry Iv Test 1-Section B: Date: 28 August 2017Document3 pagesAnalytical Chemistry Iv Test 1-Section B: Date: 28 August 2017ashNo ratings yet
- Final 2018 - Eng - Con SolucDocument11 pagesFinal 2018 - Eng - Con SolucCezar AlexandruNo ratings yet
- Course Code: 4360504Document9 pagesCourse Code: 4360504Abhishek ThummarNo ratings yet
- Fundamentals of Automation Technology 20EE43P PortfolioDocument35 pagesFundamentals of Automation Technology 20EE43P PortfolioThanmay JSNo ratings yet
- Electropneumatic and Hydraulic DNT241: Experiment No. 3Document13 pagesElectropneumatic and Hydraulic DNT241: Experiment No. 3Alex ZXNo ratings yet
- CHT302 - Ktu QbankDocument8 pagesCHT302 - Ktu Qbanknaagin12300No ratings yet
- Conceptual Design and Analysis Methodology For Crystalliza 2002 Fluid PhaseDocument21 pagesConceptual Design and Analysis Methodology For Crystalliza 2002 Fluid PhaseAnonymous ypVNIINo ratings yet
- Book - Microwave Green ExtractionDocument150 pagesBook - Microwave Green ExtractionAlpha AlkaffNo ratings yet
- Chemical EngineeringDocument9 pagesChemical EngineeringanushafiNo ratings yet
- Oiml TC17 SC2 N3 2007Document25 pagesOiml TC17 SC2 N3 2007acanis1016No ratings yet
- CHT397 - Ktu QbankDocument7 pagesCHT397 - Ktu QbankJo MonNo ratings yet
- Lab Report 2 Composite MaterialsDocument30 pagesLab Report 2 Composite MaterialsartynskuNo ratings yet
- Southon 2020Document4 pagesSouthon 2020Ensieh FarzanehNo ratings yet
- Final Report On The CIPM Air Speed Key Comparison (CCM - FF-K3)Document21 pagesFinal Report On The CIPM Air Speed Key Comparison (CCM - FF-K3)fabiouverneyNo ratings yet
- FinalDocument4 pagesFinalSimge DemirNo ratings yet
- 1 s2.0 S1026918522000476 MainDocument12 pages1 s2.0 S1026918522000476 MainNórida Pájaro GómezNo ratings yet
- ManualCEE2020v3 PDFDocument48 pagesManualCEE2020v3 PDFJunaid Ijaz GurmaniNo ratings yet
- Dhar Anuj - India 39 S Biggest Cover-Up 2012 AnujDocument49 pagesDhar Anuj - India 39 S Biggest Cover-Up 2012 AnujAbhishek PaloNo ratings yet
- Group B2 - CSTR - Flow RateDocument7 pagesGroup B2 - CSTR - Flow RateMimi Sharina HassanNo ratings yet
- Industrial Chemistry Course Code: C4320501: Page 1 of 11Document11 pagesIndustrial Chemistry Course Code: C4320501: Page 1 of 11tdhyey63No ratings yet
- PDocument2 pagesPrajpd28No ratings yet
- IMA Questions PaperDocument17 pagesIMA Questions PaperAj ShindeNo ratings yet
- Analytical Chemistry I - Lab Manual - 12102022Document39 pagesAnalytical Chemistry I - Lab Manual - 12102022zahin oktoNo ratings yet
- M Pharm PDFDocument49 pagesM Pharm PDFkothi hemaraniNo ratings yet
- R Reeggaarrddiinngg Tthhee Eexxaam Miinnaattiioonn: GDD ? H@Qka A9Fk KMJ? GFK H9Cakl9FDocument3 pagesR Reeggaarrddiinngg Tthhee Eexxaam Miinnaattiioonn: GDD ? H@Qka A9Fk KMJ? GFK H9Cakl9FAamir HamaadNo ratings yet
- Methodology & Acknowledgment (Thesis)Document1 pageMethodology & Acknowledgment (Thesis)anissfarhanaaNo ratings yet
- Group Assignment Organic ChemistryDocument1 pageGroup Assignment Organic ChemistryanissfarhanaaNo ratings yet
- AbstractDocument1 pageAbstractanissfarhanaaNo ratings yet
- Case Study (ABSTRACT)Document1 pageCase Study (ABSTRACT)anissfarhanaaNo ratings yet
- Template Full Paper SeminarDocument2 pagesTemplate Full Paper SeminaranissfarhanaaNo ratings yet
- Scope of Engineering EthicsDocument3 pagesScope of Engineering EthicsanissfarhanaaNo ratings yet
- Ac LR 2Document12 pagesAc LR 2Fadhlin SakinahNo ratings yet
- Ac LR 1Document13 pagesAc LR 1Anonymous d3Zx5r4jX100% (1)
- Separating Vaporized Decomposition: Pre Laboratory QuestionDocument7 pagesSeparating Vaporized Decomposition: Pre Laboratory QuestionanissfarhanaaNo ratings yet
- Ac LR 3Document15 pagesAc LR 3anissfarhanaaNo ratings yet
- Abstract EXP PRESSUREDocument1 pageAbstract EXP PRESSUREanissfarhanaaNo ratings yet
- Concl & Recomm EXP LEVEL FLOWDocument1 pageConcl & Recomm EXP LEVEL FLOWanissfarhanaaNo ratings yet
- Chap1 EconometricsDocument36 pagesChap1 EconometricsLidiya wodajenehNo ratings yet
- PPTDocument1 pagePPTawdqNo ratings yet
- V. Prabhakaran (Ed.) - Methodology of Philosophy (2011)Document57 pagesV. Prabhakaran (Ed.) - Methodology of Philosophy (2011)Rockandopera100% (3)
- Matched Pair+Hypothesis+TestingDocument8 pagesMatched Pair+Hypothesis+TestingAmarnathMaitiNo ratings yet
- How To Write A Null Hypothesis in A Research PaperDocument6 pagesHow To Write A Null Hypothesis in A Research PaperefdrkqkqNo ratings yet
- GE JD21 Dr. Sugay J.Document6 pagesGE JD21 Dr. Sugay J.Daisy Marie OrolaNo ratings yet
- Onion CellDocument5 pagesOnion CellNiah ComendadorNo ratings yet
- Simran Yadav RMDocument76 pagesSimran Yadav RMGAURAVNo ratings yet
- ECON2046 - 2005 Week 6 Lecture 9 Slides 1 Per PageDocument26 pagesECON2046 - 2005 Week 6 Lecture 9 Slides 1 Per PagedsascsNo ratings yet
- AP Stats Detailed SyllabusDocument16 pagesAP Stats Detailed SyllabusNaresh KumarNo ratings yet
- 3rd Quarter Examination in 3isDocument2 pages3rd Quarter Examination in 3iseliana tm31100% (2)
- MTE3105 Pengujian Hipotesis Khi Kuasa Dua 2Document32 pagesMTE3105 Pengujian Hipotesis Khi Kuasa Dua 2Sitherrai ParamananthanNo ratings yet
- DBA Doctoral Study ProspectusDocument4 pagesDBA Doctoral Study ProspectusDr. Stacey Q WhitmoreNo ratings yet
- E2857-11 Analytical Validation Method PDFDocument5 pagesE2857-11 Analytical Validation Method PDFswapon kumar shillNo ratings yet
- How To Write Chapter 3Document8 pagesHow To Write Chapter 3Ciann Ghester F. RentoriaNo ratings yet
- Panel Stochastic Frontier Models With Endogeneity in Stata: Mustafa U. KarakaplanDocument13 pagesPanel Stochastic Frontier Models With Endogeneity in Stata: Mustafa U. KarakaplanMireya Ríos CaliNo ratings yet
- 10 Challenging Problems in Data Mining ResearchDocument8 pages10 Challenging Problems in Data Mining Researchabhi_cool25No ratings yet
- Z Test ThesisDocument5 pagesZ Test Thesisafloihzesdawig100% (2)
- IBMDocument4 pagesIBMrodrigo ortizNo ratings yet
- Individualogist (Review 2021) by JamDPFreeDocument16 pagesIndividualogist (Review 2021) by JamDPFreeJamel CastilloNo ratings yet
- Section and SolutionDocument4 pagesSection and SolutionFiromsaNo ratings yet
- Richard Freeman, Antony Stone Study Skills For Psychology Succeeding in Your Degree Sage Study Skills SeriesDocument176 pagesRichard Freeman, Antony Stone Study Skills For Psychology Succeeding in Your Degree Sage Study Skills Seriesashish1981No ratings yet
- Measuring The Stray Light Performance of UV-visible SpectrophotometersDocument4 pagesMeasuring The Stray Light Performance of UV-visible SpectrophotometersDiego Fernado AvendañoNo ratings yet
- Weekly Quiz 2 Predictive Modeling Logistic Regression PDFDocument3 pagesWeekly Quiz 2 Predictive Modeling Logistic Regression PDFsavita bohra100% (1)
- Lecture 1 - Introduction - Statistic BusinessDocument6 pagesLecture 1 - Introduction - Statistic BusinessNguyễn Quang AnhNo ratings yet
- Multiple Regression ProjectDocument10 pagesMultiple Regression Projectapi-27739305433% (3)
- Rev Insurance Business ReportDocument4 pagesRev Insurance Business ReportPratigya pathakNo ratings yet
- Research Chapter 3Document3 pagesResearch Chapter 3GG wp50% (2)
- Stat PPT 2Document17 pagesStat PPT 2Ma'am YemaNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet