Professional Documents
Culture Documents
Exercise 2
Uploaded by
Anant Dwivedi0 ratings0% found this document useful (0 votes)
30 views1 pageOriginal Title
Exercise_2
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
30 views1 pageExercise 2
Uploaded by
Anant DwivediCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
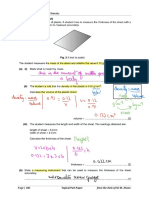
Page # 10 Solutions Slot – 3 (Chemistry)
EXERCISE – II SUBJECTIVE LEVEL-I
1. A, C 7. C
H2(g) 2H(g)
2. C, D end of the mic
H>0 High Temp
3. B, C ng>0 low presses
8. C, D
4. D Introduction of inet gas at constant
Pressure loill the volume & the pressre
K P2 1 1 of gases of equilibrium
H
ln K = R T – T
p1 1 2 equilibrium shifted forwasd
9. C, D
H 1 1
NaNO3 (s) NaNO2(s) + O2(g)
ln 4 = –
8.314 298 313
H > 0 end of thermic
H° = 71.6 kJ High temperature forwendren
5. A, C 1000 pressure
NH2COONH4 (s) 2NH3 9 + CO2(g)
High pressure Reverse reachron
2x x
12. B, C
(2x)2(x) = 2.92×10–5
Exothermic low temp
2.92 forward
x3 = 10–5
4 High temp backward
Ptotal = 3x = 0.0582
1
6. 2H2(g)+O2 2H2O(g) K
1
2CO2 2CO(g) + 2()O2 K2
2H2(g) + 2CO(g) 2H2O(g) + 2CO(g)
K2
K1
H2(g) + CO2(g) H2O(g)+CO(g)
K2
K= K1
K = 2.58
394 - Rajeev Gandhi Nagar Kota, Ph. No. 0744-2209671, 93141-87482, 93527-21564
IVRS No. 0744-2439051, 0744-2439052, 0744-2439053 www.motioniitjee.com, email-info@motioniitjee.com
You might also like
- 3 2 EnthalpyDocument14 pages3 2 EnthalpymahmoudNo ratings yet
- Media 1505143131 918696Document18 pagesMedia 1505143131 918696happyNo ratings yet
- Practice Quiz 1 ANSWER KEY 2017Document2 pagesPractice Quiz 1 ANSWER KEY 2017Frezeil RocheNo ratings yet
- Adiabatic Flame Temperature Example ProblemDocument2 pagesAdiabatic Flame Temperature Example ProblemdarthmaniNo ratings yet
- Gcesoln 6Document26 pagesGcesoln 6api-3734333100% (1)
- Equilibrium KeyDocument4 pagesEquilibrium KeySunnyNo ratings yet
- The Basic Problems With SolutionsDocument6 pagesThe Basic Problems With SolutionsManvitha ReddyNo ratings yet
- Combustion: Flame Theory and Heat Produced: Arthur Anconetani Oscar Castillo Everett HendersonDocument29 pagesCombustion: Flame Theory and Heat Produced: Arthur Anconetani Oscar Castillo Everett HendersonTommy Cha Yee WenNo ratings yet
- PHYSICAL CHEMISTRY - 30-07 13th ObjectiveDocument5 pagesPHYSICAL CHEMISTRY - 30-07 13th ObjectiveRaju SinghNo ratings yet
- NEET States of Matter - Gases and Liquid Important QuestionsDocument28 pagesNEET States of Matter - Gases and Liquid Important QuestionsUjjwal KumarNo ratings yet
- Equilibrium-Constant NOTESDocument5 pagesEquilibrium-Constant NOTESAlex Jethro TigoyNo ratings yet
- Adiabatic Reactor TemperatureDocument4 pagesAdiabatic Reactor TemperatureAgam HanasichulaNo ratings yet
- Bernoulli Equation Part 2Document26 pagesBernoulli Equation Part 2Nabayan SahaNo ratings yet
- Kinetic (Graphical Analysis) 21Document3 pagesKinetic (Graphical Analysis) 21滾滾滾滾滾滾No ratings yet
- QuizDocument1 pageQuizRoxette RoseteNo ratings yet
- Solutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 2: (Chemistry)Document10 pagesSolutions To Mock IIT Advanced Test - 1/JEE-2018/Paper - 2: (Chemistry)rajeshNo ratings yet
- Chemical KineticsDocument20 pagesChemical Kineticsvijaylakshmi0727No ratings yet
- Abril-Duhaylongsod-Baluran - Modelling ProjectDocument13 pagesAbril-Duhaylongsod-Baluran - Modelling ProjectKim Rohn AbrilNo ratings yet
- Kinetics Practice QuestionDocument6 pagesKinetics Practice Question장채윤No ratings yet
- Home Assignment 1 - Ersa Berliana - 04211941000041Document6 pagesHome Assignment 1 - Ersa Berliana - 04211941000041roberto luckyNo ratings yet
- Unit # 07 (Part - I) : Chemical Equilibrium Exercise # 1Document6 pagesUnit # 07 (Part - I) : Chemical Equilibrium Exercise # 11234vishal mimaniNo ratings yet
- A-Levels Chem NotesDocument22 pagesA-Levels Chem Notesd-fbuser-69634921No ratings yet
- CHEMISTRY - (13th) (OIP) Paper-2Document6 pagesCHEMISTRY - (13th) (OIP) Paper-2Raju SinghNo ratings yet
- Thermodynamic TestDocument3 pagesThermodynamic TestRk kashyapNo ratings yet
- Chapter 17 - Rev PDFDocument13 pagesChapter 17 - Rev PDFalaa al sahmaraniNo ratings yet
- 04 GR Elasticity Heat Solution PDFDocument4 pages04 GR Elasticity Heat Solution PDFHappy SinghNo ratings yet
- Thermo Chemistry Exercise # 1: FCH fCO fHODocument4 pagesThermo Chemistry Exercise # 1: FCH fCO fHO10A31 Irfan HashmiNo ratings yet
- EntropiaDocument3 pagesEntropiaMargarida PereiraNo ratings yet
- Activity Sheet - Thermochemistry With Chemical Kinetics PDFDocument2 pagesActivity Sheet - Thermochemistry With Chemical Kinetics PDFAirah Joyce ParedesNo ratings yet
- Instructions: CHE 314 - Heat Transfer Final Exam (Fall 2018), Dec. 18, 9:00am-11:00am: PavilionDocument8 pagesInstructions: CHE 314 - Heat Transfer Final Exam (Fall 2018), Dec. 18, 9:00am-11:00am: PavilionAkib ImtihanNo ratings yet
- Equilibrium ShiftDocument2 pagesEquilibrium ShiftCatalina PerryNo ratings yet
- Principles of Heat Transfer 8th Edition Kreith Solutions ManualDocument25 pagesPrinciples of Heat Transfer 8th Edition Kreith Solutions ManualBrianJimenezanco97% (37)
- Nov 2004 P3Document9 pagesNov 2004 P3Ying LiangNo ratings yet
- Chapter 4 - Section B - Non-Numerical Solutions: 4.5 For Consistency With The Problem Statement, We Rewrite Eq. (4.8) AsDocument2 pagesChapter 4 - Section B - Non-Numerical Solutions: 4.5 For Consistency With The Problem Statement, We Rewrite Eq. (4.8) AsNic BlandoNo ratings yet
- Dwnload Full Principles of Heat Transfer 8th Edition Kreith Solutions Manual PDFDocument22 pagesDwnload Full Principles of Heat Transfer 8th Edition Kreith Solutions Manual PDFcherylmcdonaldbksentqwrm100% (10)
- ZZ Chem 1202 Test 1 New Formula SheetDocument5 pagesZZ Chem 1202 Test 1 New Formula Sheetavanit1No ratings yet
- Heat Capacity Ratio For Gases (G) : 2pa3 Experiment 3ADocument16 pagesHeat Capacity Ratio For Gases (G) : 2pa3 Experiment 3AMahdi GharibNo ratings yet
- Chapter 1 Solutions DetailedDocument30 pagesChapter 1 Solutions DetailedYeonjae JeongNo ratings yet
- CH1104 Chapter 9Document68 pagesCH1104 Chapter 9Chuah Chong YangNo ratings yet
- Thermodynamics 2Document22 pagesThermodynamics 2Gowri ShankarNo ratings yet
- Greg Heat Loss To SurroundingDocument6 pagesGreg Heat Loss To SurroundingMarta BaptistaNo ratings yet
- Hesss LawDocument2 pagesHesss LawBiblee ChasNo ratings yet
- JEE Advanced 2022 Solved Paper 1Document12 pagesJEE Advanced 2022 Solved Paper 1Gaurav KumarNo ratings yet
- Chem Eqb.Document5 pagesChem Eqb.Preethi SekarNo ratings yet
- Pre Heater Design CalculationsDocument4 pagesPre Heater Design CalculationsFahad KhokharNo ratings yet
- Test3 ch14 ThermodynamicsPractice-answers-markedDocument7 pagesTest3 ch14 ThermodynamicsPractice-answers-markedKalina EllisNo ratings yet
- Equilibrium DPP 014728Document11 pagesEquilibrium DPP 014728Yash MalviyaNo ratings yet
- ThermodynamicsDocument7 pagesThermodynamicscrazy boyNo ratings yet
- Gen-1 JEE Main-7 - JEE 2024 - SolutionDocument18 pagesGen-1 JEE Main-7 - JEE 2024 - SolutionKunjesh Raushan SinghNo ratings yet
- 2-1 Chemical Equilibrium 2-09-2019Document114 pages2-1 Chemical Equilibrium 2-09-2019carlos lara rodriguezNo ratings yet
- PED Mid Term Formula SheetDocument4 pagesPED Mid Term Formula SheetAvinash Subramanian S 19BCM0079No ratings yet
- PHYSICAL CHEMISTRY - (13th) TEST-6Document5 pagesPHYSICAL CHEMISTRY - (13th) TEST-6Raju SinghNo ratings yet
- Chemical Equilibrium Assig (Ans) 24 03 21Document6 pagesChemical Equilibrium Assig (Ans) 24 03 21Rushikesh ThoratNo ratings yet
- DPP-5 - Student Copy (Chemical Equlibrium)Document4 pagesDPP-5 - Student Copy (Chemical Equlibrium)prashantyadavpky07No ratings yet
- Ame Homework Solutions 1 Fall 2011Document33 pagesAme Homework Solutions 1 Fall 2011Ťhåŕüñ Kūmæř GøwđNo ratings yet
- Hydrology - Collection of Formulas-: (Aid For The Exam and The Assignments)Document21 pagesHydrology - Collection of Formulas-: (Aid For The Exam and The Assignments)HectorAbelAstocazaGNo ratings yet
- Final Exam - TA Class - Updated 12 15 2022Document70 pagesFinal Exam - TA Class - Updated 12 15 2022tran huyNo ratings yet
- Chemistry NotesDocument50 pagesChemistry NotesSinenhlahla ThethwayoNo ratings yet
- Stream 18: H ∆ Ĥ f (l) + Cp Dt + Δp ṽDocument20 pagesStream 18: H ∆ Ĥ f (l) + Cp Dt + Δp ṽAhmed Qutb AkmalNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Allen: Subjective AssignmentDocument5 pagesAllen: Subjective AssignmentAnant DwivediNo ratings yet
- Xercise: Advanced Subjective QuestionsDocument2 pagesXercise: Advanced Subjective QuestionsAnant DwivediNo ratings yet
- Allen: Subjective AssignmentDocument5 pagesAllen: Subjective AssignmentAnant DwivediNo ratings yet
- Allen: Subjective AssignmentDocument2 pagesAllen: Subjective AssignmentAnant DwivediNo ratings yet
- Allen: Subjective AssignmentDocument2 pagesAllen: Subjective AssignmentAnant DwivediNo ratings yet
- Xercise: Jee ProblemsDocument2 pagesXercise: Jee ProblemsAnant DwivediNo ratings yet
- Xercise: Multiple Correct (Objective Questions)Document2 pagesXercise: Multiple Correct (Objective Questions)Anant DwivediNo ratings yet
- Xercise: Hints & SolutionsDocument3 pagesXercise: Hints & SolutionsAnant DwivediNo ratings yet
- Exercise 3Document3 pagesExercise 3Anant DwivediNo ratings yet
- SolutionsDocument17 pagesSolutionsAnant DwivediNo ratings yet
- Mock INMO SolutionsDocument8 pagesMock INMO SolutionsAnant DwivediNo ratings yet
- Thermodynamics: Theory and Exercise BookletDocument2 pagesThermodynamics: Theory and Exercise BookletAnant DwivediNo ratings yet
- Xercise: Jee ProblemsDocument5 pagesXercise: Jee ProblemsAnant DwivediNo ratings yet
- E - Iii: XerciseDocument5 pagesE - Iii: XerciseAnant DwivediNo ratings yet
- Conants PDFDocument2 pagesConants PDFAnant DwivediNo ratings yet
- E - Iii: XerciseDocument4 pagesE - Iii: XerciseAnant DwivediNo ratings yet
- Xercise: Advanced Subjective QuestionsDocument4 pagesXercise: Advanced Subjective QuestionsAnant DwivediNo ratings yet
- Theory and Exercise BookletDocument2 pagesTheory and Exercise BookletAnant DwivediNo ratings yet
- Answer Key ThermodynamicsDocument18 pagesAnswer Key ThermodynamicsAnant DwivediNo ratings yet
- Exercise - II: (A) Moment of InertiaDocument3 pagesExercise - II: (A) Moment of InertiaAnant DwivediNo ratings yet
- Exercise - III: (A) Moment of InertiaDocument4 pagesExercise - III: (A) Moment of InertiaAnant DwivediNo ratings yet
- Exercise - IV: C D B A ODocument3 pagesExercise - IV: C D B A OAnant DwivediNo ratings yet
- Exer 3Document1 pageExer 3Anant DwivediNo ratings yet
- Xercise: Multiple Choice QuestionsDocument2 pagesXercise: Multiple Choice QuestionsAnant DwivediNo ratings yet
- Elasticity & Thermal Expansion: XerciseDocument3 pagesElasticity & Thermal Expansion: XerciseAnant DwivediNo ratings yet
- Answer KeyDocument1 pageAnswer KeyAnant DwivediNo ratings yet
- Internal Test-I SSDocument2 pagesInternal Test-I SSBALAJI.D MCE-AP/ECENo ratings yet
- 1.5 Ratio and ProportionDocument13 pages1.5 Ratio and ProportionAin ShakilaNo ratings yet
- O Levels Physics BookDocument13 pagesO Levels Physics BookTalha Ahsan100% (1)
- Tutorial 2 Superposition and Reflection of Pulses: Instructor: Kazumi TolichDocument13 pagesTutorial 2 Superposition and Reflection of Pulses: Instructor: Kazumi TolichHydeki RyugaNo ratings yet
- 3 A) PDS - E - JER - v4.1Document4 pages3 A) PDS - E - JER - v4.1Anish KarthikeyanNo ratings yet
- Micro Motion, IncDocument2 pagesMicro Motion, IncMahmoud Abd-Elhamid Abu EyadNo ratings yet
- BH-60 Fixed Gas Detector Operation ManualDocument8 pagesBH-60 Fixed Gas Detector Operation ManualTan KokkiangNo ratings yet
- Cloud Gary - Optical Methods of Engineering Analysis (1998) PDFDocument517 pagesCloud Gary - Optical Methods of Engineering Analysis (1998) PDFDavid Alejandro TorresNo ratings yet
- Matrices and DeterminantsDocument37 pagesMatrices and DeterminantsKiran MittalNo ratings yet
- How To Download Notes Guides Past PapersDocument33 pagesHow To Download Notes Guides Past PapersHassan NaeemNo ratings yet
- Inversion of Time-Lapse Surface Gravity Data For DDocument25 pagesInversion of Time-Lapse Surface Gravity Data For DFatimah Az ZahraNo ratings yet
- UCONN - Math 3435 - Spring 2018 - Problem Set 2Document3 pagesUCONN - Math 3435 - Spring 2018 - Problem Set 2Gyebi EmmanuelNo ratings yet
- Joint Sealants For Airport Pavements: DOT/FAA/CT-94/53Document151 pagesJoint Sealants For Airport Pavements: DOT/FAA/CT-94/53Phạm Thanh PhươngNo ratings yet
- Rilem TC 162 Parte 1Document12 pagesRilem TC 162 Parte 1Scientific GamerNo ratings yet
- F1-10 Basic Hydraulics Bench - Issue 19Document12 pagesF1-10 Basic Hydraulics Bench - Issue 19adiimacNo ratings yet
- Bolted Flanged Joint Creep/Relaxation Results at High TemperaturesDocument7 pagesBolted Flanged Joint Creep/Relaxation Results at High TemperaturesjlbarretoaNo ratings yet
- OTC 27289 MS - FinalDocument19 pagesOTC 27289 MS - FinalnsaifulNo ratings yet
- Jee Main Leader SyllabusDocument1 pageJee Main Leader SyllabusMampNo ratings yet
- Aristo Science Workbook 1A (Answer) PDF Solubility EvaporationDocument1 pageAristo Science Workbook 1A (Answer) PDF Solubility Evaporationntlroblox870% (1)
- Bus Bar Design Calculations For Busduct MarsDocument8 pagesBus Bar Design Calculations For Busduct MarsKamesh1977No ratings yet
- A Note On Rakotch ContractionsDocument7 pagesA Note On Rakotch ContractionsTalha WaheedNo ratings yet
- Test-Summary Formation FactorDocument15 pagesTest-Summary Formation FactorWanucy Barroso RodriguesNo ratings yet
- Brochure Liquid Cylinders DOT 4LDocument8 pagesBrochure Liquid Cylinders DOT 4LShaheer TariqNo ratings yet
- UNIT-III Magnetic and Superconducting MaterialsDocument12 pagesUNIT-III Magnetic and Superconducting MaterialsSonu GoudNo ratings yet
- Tennessee Science Student Edition Grade 8Document1 pageTennessee Science Student Edition Grade 8Arshdeep SinghNo ratings yet
- Sa Node Action PotentialDocument2 pagesSa Node Action PotentialAljon AniesNo ratings yet
- (Chapter) Neonatal Infrared Thermography MonitoringDocument43 pages(Chapter) Neonatal Infrared Thermography MonitoringErwin SmithNo ratings yet
- (Derek Holton) A First Step To Mathematical Olympi (B-Ok - CC)Document284 pages(Derek Holton) A First Step To Mathematical Olympi (B-Ok - CC)Nita Setiawati100% (1)
- Em Ut5Document17 pagesEm Ut5david josephNo ratings yet
- Lecture 3 - Laplace Transform-Part1Document12 pagesLecture 3 - Laplace Transform-Part1John AlvarezNo ratings yet