Professional Documents
Culture Documents
General Chemistry Laboratory Results: Determination of Density
Uploaded by
Ariane0 ratings0% found this document useful (0 votes)
6 views2 pagesOriginal Title
Results-Detemination of Density
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesGeneral Chemistry Laboratory Results: Determination of Density
Uploaded by
ArianeCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
General Chemistry Laboratory
Results: Determination of Density
I. Identifying objects:
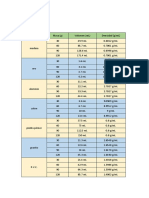
OBJECTS MASS (kg) VOLUME (L) DENSITY (kg/L) IDENTITY
A 65.14 kg 3.38 L 19.27 kg/L Gold
B 0.64 kg 0.64 L 1 kg/L Gasoline
C 4.08 kg 4.08 L 1 kg/L Ice
D 3.10 kg 3.10 L 1 kg/L Water
E 3.53 kg 1.00 L 3.53 kg/L Diamond
II. Why do objects float?
SINK or
OBJECTS MASS (g) VOLUME (ml) DENSITY (g/ml)
FLOAT?
wood 13.30 g 15.6 ml 0.853 g/ml float
aluminum 5.60 g 1.1 ml 5.1 g/ml sink
plastic 4.00 g 4.1 ml 0.58 g/ml float
lead 20.00 g 1.8 ml 11 g/ml sink
cork 4.00 g 8.1 ml 0.50 g/ml float
steel 8.30 g 1.6 ml 5.2 g/ml sink
clay 15.60 g 8.5 ml 1.8 g/ml sink
rubber 5.90 g 4.9 ml 1.2 g/ml sink
candle 10.40 g 10.5 ml 0.990 g/ml float
QUESTIONS:
1. Describe some application of density measurement? Why is it important to know?
Density measurement is used to define and describe a pure substance. It also
specifies the proportion of the binary mixture to provide information on the structure
of mixtures. Furthermore, one example of an application of density is to understand
how an object or substance floats. "Fluid motion lamp" is an example of density and
buoyancy in motion as the blobs of oozing goo or slime-like shift in up and down
movement and different forms. These lamps consist of a glass container in which a
colored oily liquid is placed, which is not mixed with water and constitutes a second
step. The composition of the oil phase is at room temperature, its density is slightly
higher than the water, so it usually rests at the bottom of the bottle. When the lamp is
turned on it will produce heat that comes from the light bulb that makes the oil heat
up and suddenly reduces its density to a value below the water, causing the oil to rise
to the top of the container with up and down movement. And when the lamp is cool
down it will get back to its original place.
In conclusion, density measurement is important because we can find the
volume of a substance like which liquid is a heavier flow or thicker molecules in it.
Thus, all substances tend to expand as they are heated, causing the same mass to
occupy a greater volume and lower the density.
You might also like
- UNIT 1 - Assignment 2 - Significant Figures and Density - Answer KeyDocument1 pageUNIT 1 - Assignment 2 - Significant Figures and Density - Answer KeyRoseman TumaliuanNo ratings yet
- DensityDocument15 pagesDensityapi-286079895No ratings yet
- DensityLabSE Key PDFDocument5 pagesDensityLabSE Key PDFZamya Livingston27% (11)
- 3 Lab ReportDocument4 pages3 Lab ReportSasquatchCornNo ratings yet
- Student Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumeDocument6 pagesStudent Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumeOctoso AishaNo ratings yet
- Student Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumeDocument6 pagesStudent Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumePaul CornejoNo ratings yet
- Density Workshop PDFDocument1 pageDensity Workshop PDFJuana Valentina Londoño BritoNo ratings yet
- What Is DensityDocument5 pagesWhat Is DensityAnggi HerdiansyahNo ratings yet
- A. Regular Solid:: 1 21.5cm 11.7cm 3cm 754.65 CC 1100g 1.457 G/CC 2 21.5cm 11.8cm 3.4cm 862.58 CC 1100g 1.275 G/CCDocument4 pagesA. Regular Solid:: 1 21.5cm 11.7cm 3cm 754.65 CC 1100g 1.457 G/CC 2 21.5cm 11.8cm 3.4cm 862.58 CC 1100g 1.275 G/CCJaypee MasculinoNo ratings yet
- DENSITY My Teacher Once Asked Me, "Is Wool Heavier orDocument4 pagesDENSITY My Teacher Once Asked Me, "Is Wool Heavier orChristison AlorciousNo ratings yet
- Chemistry Lab Report-2.Docx Pre FinalDocument10 pagesChemistry Lab Report-2.Docx Pre FinalGairus John BotejaNo ratings yet
- Laboratorio de FisicaDocument1 pageLaboratorio de FisicaDaniel SarmientoNo ratings yet
- Experiment 1Document16 pagesExperiment 1Alyssa Celine RonquilloNo ratings yet
- Fluid Mechanics - Unit 1 - Justine T. SaldivarDocument10 pagesFluid Mechanics - Unit 1 - Justine T. SaldivarJustine Tizon SaldivarNo ratings yet
- Exp. No 2. Measurement of Density of An Solid ObjectDocument13 pagesExp. No 2. Measurement of Density of An Solid ObjectmonNo ratings yet
- Lab ReportDocument2 pagesLab ReportAariv IyengarNo ratings yet
- SustanciaDocument1 pageSustanciaSERGIO YAVO CHOQUENo ratings yet
- 1.6b DIMENSIONAL ANALYSIS 1Document25 pages1.6b DIMENSIONAL ANALYSIS 1Guile MacaNo ratings yet
- IodofomDocument9 pagesIodofomrulmadhaniNo ratings yet
- Lab Report: Experiment: 04: ObjectiveDocument4 pagesLab Report: Experiment: 04: Objectivenadeem ranaNo ratings yet
- Informe AnalisisDocument3 pagesInforme AnalisisJesus Daniel OrtizNo ratings yet
- Density Worksheet 1Document4 pagesDensity Worksheet 1queenjoseNo ratings yet
- Activity No 1Document5 pagesActivity No 1cr241188No ratings yet
- Exploration - Density LaboratoryDocument5 pagesExploration - Density LaboratoryCARYS BROWNNo ratings yet
- Q1 Week 3 DensityDocument7 pagesQ1 Week 3 Densityyesha arlertNo ratings yet
- Determination of DensitiesDocument4 pagesDetermination of DensitiesJulian CarantoNo ratings yet
- Student Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumeDocument5 pagesStudent Exploration: Density Laboratory: Vocabulary: Buoyancy, Density, Graduated Cylinder, Mass, Matter, Scale, VolumeOctoso AishaNo ratings yet
- Bioplastic From Plant Starch AnalysisDocument3 pagesBioplastic From Plant Starch AnalysisViene XeloNo ratings yet
- Gayo - Activity 2Document5 pagesGayo - Activity 2YEEHSHIN JILL GAYONo ratings yet
- Makenzie Lippen - Copy of DensityLabSEDocument5 pagesMakenzie Lippen - Copy of DensityLabSEMakenzie LippenNo ratings yet
- Density: Measuring The Density of MatterDocument12 pagesDensity: Measuring The Density of MatterŽeljko PosavecNo ratings yet
- Density: Measuring The Density of MatterDocument13 pagesDensity: Measuring The Density of MatterDaphne ComendadorNo ratings yet
- Density: Measuring The Density of MatterDocument12 pagesDensity: Measuring The Density of MatterŽeljko PosavecNo ratings yet
- Chapter 1 Measurements: 1.7 DensityDocument21 pagesChapter 1 Measurements: 1.7 DensityUli Gi BustilloxNo ratings yet
- CHEMLAB-Prelims ProjectDocument5 pagesCHEMLAB-Prelims ProjectDimayacyac, Ziara Jane S.No ratings yet
- What Makes A Ship To FloatDocument40 pagesWhat Makes A Ship To FloatAndre MantovaNo ratings yet
- Activity 1.11Document1 pageActivity 1.11jueliiya0% (1)
- Review Sheet - Chemistry, Level 3 - Ch. 15: SolutionsDocument3 pagesReview Sheet - Chemistry, Level 3 - Ch. 15: Solutionsjehov canteraNo ratings yet
- Chem 1701-04 Lab 1 Greg Houselander 100695767Document4 pagesChem 1701-04 Lab 1 Greg Houselander 100695767api-405158228No ratings yet
- Lab V. Density Determination: D M V G? M V × V M D × V ML ×25 ML 67.5 GDocument2 pagesLab V. Density Determination: D M V G? M V × V M D × V ML ×25 ML 67.5 GLiliana PerezNo ratings yet
- Module 5 Chem LabDocument8 pagesModule 5 Chem LabAivan NovillaNo ratings yet
- Ayala JohnFelix E-Guidesheet2Document6 pagesAyala JohnFelix E-Guidesheet2John Felix PalenciaNo ratings yet
- Density Worksheet ChallengingDocument1 pageDensity Worksheet Challengingjavier2110martinezNo ratings yet
- Lapres KF - Massa Jenis Solid Dan LiquidDocument5 pagesLapres KF - Massa Jenis Solid Dan LiquidTriyaldi MaulanaNo ratings yet
- Mass and Density: Have Fun !!!Document26 pagesMass and Density: Have Fun !!!Nafis SprataNo ratings yet
- Msds SPDocument9 pagesMsds SPPaulie Salgado DelaParraNo ratings yet
- Density Word Problems: Show Your Work!Document2 pagesDensity Word Problems: Show Your Work!Suvarnia NaidooNo ratings yet
- Experiment No. 1 - Determination of DensitiesDocument6 pagesExperiment No. 1 - Determination of DensitiesKevin F. CortesNo ratings yet
- Jurnal Percobaan Manufacture of Cis and Trans-Potassium Dioxalatodiacochromat (III)Document3 pagesJurnal Percobaan Manufacture of Cis and Trans-Potassium Dioxalatodiacochromat (III)AnnisaNo ratings yet
- EXP1POSTLABDocument13 pagesEXP1POSTLABGiane MagimotNo ratings yet
- Measurement LabDocument4 pagesMeasurement LabHenessa GumiranNo ratings yet
- De La Salle Health Sciences Institute College of Medical Radiation TechnologyDocument5 pagesDe La Salle Health Sciences Institute College of Medical Radiation TechnologyBern Austin EsguerraNo ratings yet
- Densidad G/CM Oz/gal Lb/pulg KG/DMDocument5 pagesDensidad G/CM Oz/gal Lb/pulg KG/DMManuel LantiguaNo ratings yet
- Den The DensityDocument5 pagesDen The DensityKenishaHughesNo ratings yet
- Density NotesDocument3 pagesDensity NotesAngel PeayNo ratings yet
- An Introduction To Density: by Helen Hanson & John MacalusoDocument23 pagesAn Introduction To Density: by Helen Hanson & John MacalusoXerylNo ratings yet
- ACTIVITY 5.docxPCAL POST LABDocument7 pagesACTIVITY 5.docxPCAL POST LABAbuan Kristine AprilNo ratings yet
- Watkins FactorDocument5 pagesWatkins Factorsabo6181No ratings yet
- Learning Advanced Chemistry: Quarter 2 - Week 4 and 5Document7 pagesLearning Advanced Chemistry: Quarter 2 - Week 4 and 5Aliah Jeonelle RamosNo ratings yet
- General Chemistry Laboratory Classification of Solids ResultsDocument3 pagesGeneral Chemistry Laboratory Classification of Solids ResultsAriane100% (1)
- General Chemistry Laboratory Gas Laws ResultsDocument2 pagesGeneral Chemistry Laboratory Gas Laws ResultsArianeNo ratings yet
- Practice Exercise-IMF and Solids and LiquidsDocument2 pagesPractice Exercise-IMF and Solids and LiquidsArianeNo ratings yet
- Results-Properites of LiquidDocument5 pagesResults-Properites of LiquidArianeNo ratings yet
- General Chemistry Laboratory Detection of Elements in Organic CompoundsDocument1 pageGeneral Chemistry Laboratory Detection of Elements in Organic CompoundsArianeNo ratings yet
- General Chemistry Laboratory Test For Functional GroupsDocument1 pageGeneral Chemistry Laboratory Test For Functional GroupsArianeNo ratings yet
- Practice Exercise-Properties and ReactionsDocument2 pagesPractice Exercise-Properties and ReactionsArianeNo ratings yet
- General Chemistry Laboratory Chemical Reactions Results: Reaction Observations Balanced Chemical Equation Type of Chemical ReactionDocument2 pagesGeneral Chemistry Laboratory Chemical Reactions Results: Reaction Observations Balanced Chemical Equation Type of Chemical ReactionArianeNo ratings yet
- Practice Exercise-Properties and ReactionsDocument2 pagesPractice Exercise-Properties and ReactionsArianeNo ratings yet
- Results - Atomic Structure, Atomic Mass and IsotopesDocument2 pagesResults - Atomic Structure, Atomic Mass and IsotopesArianeNo ratings yet
- General Chemistry Lecture Practice Exercise: Periodic Table and Periodic TrendsDocument1 pageGeneral Chemistry Lecture Practice Exercise: Periodic Table and Periodic TrendsArianeNo ratings yet
- Results-Separation of MixturesDocument1 pageResults-Separation of MixturesArianeNo ratings yet
- Nitoflor EPU100Document4 pagesNitoflor EPU100Allan DerickNo ratings yet
- Test QuestionsDocument2 pagesTest QuestionsMOHANAPRIYANo ratings yet
- Heat Exchangers ReportDocument15 pagesHeat Exchangers ReportBigNo ratings yet
- 5 Chapter 4Document15 pages5 Chapter 4azizNo ratings yet
- Growth and Characterization of L-Alanine Potassium Nitrate Single Crystals For Nonlinear Optical ApplicationsDocument5 pagesGrowth and Characterization of L-Alanine Potassium Nitrate Single Crystals For Nonlinear Optical ApplicationsPalaniswamy SankariahNo ratings yet
- USP Method For HPLC: Analysis of MethotrexateDocument2 pagesUSP Method For HPLC: Analysis of MethotrexateIsaac GuerreroNo ratings yet
- Classification of Economic: DR S e eDocument2 pagesClassification of Economic: DR S e ekishan kumarNo ratings yet
- Sds Caustic SodaDocument8 pagesSds Caustic Sodaabil khausarNo ratings yet
- New Holland TM Electrical System Duplicated From Main ManualDocument456 pagesNew Holland TM Electrical System Duplicated From Main ManualRoong RuangNo ratings yet
- ASTM C 128 Standard Test Method For Density, Relative Density (Specific Gravity), and AbsorptionDocument6 pagesASTM C 128 Standard Test Method For Density, Relative Density (Specific Gravity), and AbsorptionRyan LasacaNo ratings yet
- Epoxi Resins Reactive Diluyents EPOSIR EPONACDocument8 pagesEpoxi Resins Reactive Diluyents EPOSIR EPONACfatemeh.ahmadkhaniNo ratings yet
- Risks and Safety Measures Intig Welding ProcessDocument5 pagesRisks and Safety Measures Intig Welding ProcessSeminarski radoviNo ratings yet
- Exceptions To The Octet Rule: Molecules With Electron-Deficient AtomsDocument22 pagesExceptions To The Octet Rule: Molecules With Electron-Deficient AtomsJohn RammNo ratings yet
- Simulation of Kaduna Refining and Petrochemical Company (KRPC) Crude Distillation Unit (CDU I) Using HysysDocument7 pagesSimulation of Kaduna Refining and Petrochemical Company (KRPC) Crude Distillation Unit (CDU I) Using HysysAdhityaEkoBagusNo ratings yet
- 022 - 01 - 03 - Fuel GaugeDocument2 pages022 - 01 - 03 - Fuel GaugeJaucafoNo ratings yet
- The Viability of Snail Shell Suso As An Additive in Making Cemented Pots Final DaftDocument29 pagesThe Viability of Snail Shell Suso As An Additive in Making Cemented Pots Final DaftRhaziela Eunika MalabagNo ratings yet
- Australia To See Fastest Energy Transition in The World Due To - 2020 - Focus oDocument1 pageAustralia To See Fastest Energy Transition in The World Due To - 2020 - Focus oBagoes IdchaNo ratings yet
- Kemtek Ni - 512: Semi Bright Electroless Nickel ProcessDocument4 pagesKemtek Ni - 512: Semi Bright Electroless Nickel ProcessAbbas RangoonwalaNo ratings yet
- Engineering Project Proposal by SlidesgoDocument10 pagesEngineering Project Proposal by SlidesgoMrtfthNo ratings yet
- Completion Fluid Services Liquid Viscosifier: DescriptionDocument2 pagesCompletion Fluid Services Liquid Viscosifier: DescriptionpaimanNo ratings yet
- Isobutylene: CAS N°: 115-11-7Document102 pagesIsobutylene: CAS N°: 115-11-7javNo ratings yet
- Cambridge IGCSE™: Chemistry 0620/52Document8 pagesCambridge IGCSE™: Chemistry 0620/52Sridharan VijayalakshmiNo ratings yet
- Screw Compressors ReviewDocument19 pagesScrew Compressors ReviewCarlos Maldonado AlmeidaNo ratings yet
- PhysicsDocument63 pagesPhysicsmsrsg25% (4)
- Heat FlowDocument22 pagesHeat FlowIshita MongaNo ratings yet
- Fluid I - Lec 3 and 4 - ProductionDocument34 pagesFluid I - Lec 3 and 4 - Productionamr mohamedNo ratings yet
- Zainal (2001) - Prediction of Performance of A Downdraft Gasifier Using Equilibrium Modeling For Different Biomass MaterialsDocument17 pagesZainal (2001) - Prediction of Performance of A Downdraft Gasifier Using Equilibrium Modeling For Different Biomass MaterialsAbraham AvNo ratings yet
- Drug Testing MethodsDocument35 pagesDrug Testing Methodswilly irawanNo ratings yet
- Theories On The Origin of The Solar System: By: Cuerpo, L.And Francisco, ADocument21 pagesTheories On The Origin of The Solar System: By: Cuerpo, L.And Francisco, ARichell G.No ratings yet
- Bai Tap Tieng Anh 7 Bai 10Document10 pagesBai Tap Tieng Anh 7 Bai 10Hươngg NguyễnnNo ratings yet