Professional Documents
Culture Documents
Diagnosis of Internal Parasites: Today's Technician
Uploaded by
MonicaCeciliaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Diagnosis of Internal Parasites: Today's Technician
Uploaded by
MonicaCeciliaCopyright:
Available Formats
Peer reviewed Today’s Technician
diagnosis of internal Parasites
Oreta M. Samples, RVT, MPH, DHSc
T
he diagnosis of internal parasites in companion animals GASTROINTESTINAL ANALYSIS
continues to evolve. Efficient methods that allow clini- The gastrointestinal (GI) tract plays host to a
cians to diagnose infections more quickly and imple- variety of helminths and protozoan parasites.
ment treatment earlier have helped pets live longer, health- Because these parasites may mature and repro-
ier lives. Because some internal parasites spread zoonotic duce in different areas of the GI system, there is
disease, such advances also help protect owners. no single best method for ova, larvae, or adult
identification.
PARASITE CLASSIFICATION & IDENTIFICATION Likewise, the method and choice of flotation
There are 2 major categories of internal parasites that affect solution that may be used to identify parasitic
both dogs and cats: ova, oocysts, or larvae will differ based on spe-
1. Protozoans (eg, Giardia, Toxoplasma, Tritrichomonas) cific gravity (SG) of the organism compared to
2. Helminths (eg, roundworms, hookworms, tapeworms). that of the chosen flotation solution. If protozoan
Although most parasites are associated with a particular infection of the GI tract is suspected, different
system of the body, due to life cycle differences, evidence of testing methods may be used to identify and dif-
their presence may be seen in a variety of places (Table 1, ferentiate between oocysts and trophozoites.
page 22). For this reason, more than 1 method of testing may The choice of solution as well as method of
be needed to confirm parasitic presence. In addition, atten- analysis contributes to the success or failure of
tion to detail with regard to health history, aseptic collection, accurate analysis. Table 2 (page 22) lists fecal
and processing of relevant samples as well as clinical experi- flotation solutions and the parasites identified
ence in the laboratory help determine diagnostic answers. through their use.1
PARASITOLOGY PRIMER
Protozoa Life Stages
Cyst: infectious form of many protozoan parasites
during which they are encapsulated inside a protec-
tive wall; usually found in the feces
Oocyst: encysted, highly resistant zygotic stage of
some sporozoan parasites that may remain infective
for extended periods of time
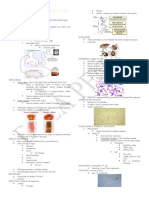
Figure 2. Ova of Fasciola
Trophozoite: active, motile feeding stage of the flagel- Figure 1. Trophozoites in hepatica, also known as
late protozoa as well as the postsporozoite state that liver tissue section common liver fluke (trematode)
is seen in some apicomplexan parasites (Figure 1)
Adult: Mature form of protozoan life capable of sexual
or asexual reproduction
Helminth Life Stages
Ova: scientific term for cells commonly referred to as
“eggs”; singular is “ovum”
Larva: independent, immature (ie, juvenile) worm that
must undergo maturation in form and size to achieve
adulthood Figure 3. Ova of Taenia Figure 4. Ova of Toxocara
species, also known as cati, also known as feline
Adult: Mature helminth life form capable of reproduction
tapeworms (cestode) roundworm (nematode)
Helminth Types
Trematode: of the class Trematoda, a parasite unique in its flat-bodied appearance (ie, liver fluke) and ability to
parasitize certain organs, such as the liver (Figure 2)
Cestode: of the class cestoda, a parasite with a ribbon-like flattened body and specialized “hold-fast” organ of
attachment at one end; commonly referred to as tapeworms (Figure 3)
Nematode: of the class nematoda, a parasite with an unsegmented and tubular, elongated body; commonly
referred to as roundworms (Figure 4)
July/August 2013 Today’s Veterinary Practice 21
| Today’s Technician
FECAL FLOTATION
TABLe 1. diagnostic Testing Organized by Fecal flotation remains the most common method to detect
Body System & Target Parasites helminth eggs and protozoan cysts.
Parasite
Diagnostic Test Flotation Solutions
Classification
Based on the SG differences of the various parts of a fecal
GasTRoinTesTinaL anaLysis sample (feces, ova, cysts, debris), the recovered eggs are lighter
Passive fecal flotation helminth eggs (ie, lower SG) than the flotation solution and will float to the
Centrifugal flotation Protozoan cysts surface. The heavier fecal matter (ie, higher SG) sinks rapidly.
nematode ova Therefore, the flotation solution must have a higher SG than
Vomit flotation the parasite eggs or cysts. It is important to note that while
(helminth)
water may often be a part of a fecal flotation solution, pure tap
Fecal sedimentation helminth ova (especially
trematode ova)
water alone cannot be used because the eggs and oocysts are
heavier, sinking in the tap water and remaining unidentified.
Baermann technique Lungworm larvae in There are several commonly used fecal flotation solutions
feces that clinicians can quickly make for clinical use (Table 3).1
Fecal culture difficult-to-identify eggs Choose solutions based on health history and expected find-
and cysts ings (Table 2).1
nematode larvae
coccidial oocysts Passive Fecal Flotation
Stained fecal smear Protozoan oocysts and Passive fecal flotation is conducted by mixing fecal matter and
trophozoites flotation solution in a small vial to form a slurry; the vial is then
BLood anaLysis filled until a positive meniscus forms (Figure 5). A cover slip is
placed over the meniscus and left undisturbed for 10 minutes,
Direct blood
which allows the eggs/cysts to float to the top (Figure 6). The
examination
heartworm microfilaria cover slip is then placed on a microscope slide and scanned at
Modified Knott’s test low power for signs of ova or larvae.2
Buffy coat method
ELISA testing heartworm antigens Centrifugal Flotation
and antibodies Centrifugal flotation “spins down” fecal debris, allowing the eggs/
cysts to float to the solution’s surface. Slide preparation is based
Stained blood smear Blood protozoa
on the type of centrifuge used (fixed head versus swing head).
URine anaLysis There are several centrifugal fecal flotation devices cur-
Urine sedimentation helminth ova rently available that include a sieve to strain the mixture,
which provides a cleaner sample for analysis; these devices
include the Fecatector (butlerschein.com), Fecalyzer (veto-
quinolusa.com), and Ovassay Plus (zoetis.com).
TABLe 2. Quick Guide to Solutions &
Target Parasites1
SOLUTION IDENTIFIABLE PARASITES
Sodium common helminths
chloride*† Protozoan ova and cysts
Sheather’s* common helminths
Protozoan ova and cysts
(particularly Cryptosporidium
oocysts)
Sodium nitrate*† common helminths Figure 5. Positive Figure 6. Cover slips placed over
Protozoan ova and cysts meniscus menisci for fecal flotation test
Zinc sulfate* common helminths
(particularly Giardia)
Protozoan ova and cysts Passive versus Centrifugal Flotation
(particularly lungworm larvae) numerous published studies have established that
Magnesium common helminths centrifugation is the preferred technique for parasite
sulfate*† Protozoan ova and cysts identification,3-6 and is, therefore, the technique rec-
*Noneffective if flukes are suspected; use of fecal
ommended by most parasitologists. The centrifugal
sedimentation is advised force allows a more rapid, efficient, and thorough
†distorts Giardia rapidly, subsequently impeding diagnosis separation of eggs/cysts and debris.
22 Today’s Veterinary Practice July/August 2013
diagnosis of internal Parasites
Today’s Technician |
The sedimentation test is most valu-
TABLe 3. Fecal Flotation Solutions1,2 able for identifying fluke ova, which have
SPECIFIC a higher SG and are larger, denser, and
SOLUTION FORMULATION heavier than other ova, and should be per-
GRAVITY
formed in suspected cases of infection.1
sodium chloride* 1.2 add table salt to boiling water until Also, because some nongastrointestinal
the salt no longer dissolves
inhabitants, such as Paragonimus kelli-
sheather’s† 1.2–1.25 454 g (1 lb) sugar + 355 mL water cotti (lung flukes), may also shed ova in
+ 6 mL 40% formaldehyde (or 1 g sputum or other internal fluids, micro-
crystalline phenol) scopic examination should be considered.2
sodium nitrate 1.2–1.33 315 g sodium nitrate: 1 L water
Zinc sulfate 1.18 386 g zinc sulfate: 1 L water BAERMANN TECHNIQUE
The Baermann testing method (often
Magnesium sulfate 1.32 350 g epson salts: 1 L water referred to as the “Baermann technique”)
*Sodium chloride solution, although widely used due to its low cost, is highly involves the concentration of nematode
corrosive and will distort eggs larvae from a fecal sample. Although this
†due to high sugar content, sheather’s solution is sticky and will attract
technique is useful for identification of
insects if not kept sealed and the surrounding area kept clean.
most nematodal larval forms, it is the gold
standard method of testing for suspected
VOMIT FLOTATION cases of lungworm infection. Table 4 (page 24) lists the most
While not common, it is possible to identify some nematode common lungworm species seen in domestic animals.7,8
ova by evaluating vomit using the same methodology as Successful larval collection relies on the fact that most
for fecal flotation. Likewise, vomit may also be scrutinized nematode species lack the ability to swim against gravity.
under a microscope to locate parasites common to the When lungworms are present in the lower respiratory tract,
stomach. larvae within the airways move with sputum to the pharynx
Vomit flotation is useful when parasites, such as where they are swallowed and later shed in the feces. Thus,
Physaloptera species or Ollulanus tricuspis, are suspected in the case of lungworms, no eggs are found in the feces,
in dogs and cats.2 Vomit flotation should also be included in only larvae.7 While there are several variations to the treat-
the initial workup for companion animals that present with ment of the sample in terms of staining and observation, the
chronic vomiting.2 basic principle for the Baermann technique remains rooted
in the simple apparatus required.
FECAL SEDIMENTATION
The fecal sedimentation test is used to detect ova that have Step by Step: Baermann Technique
a SG higher than commonly used flotation solutions and, 1. Break up fecal specimen and wrap loosely in cheese-
therefore, do not readily float. This method may also be used cloth.
for ova that will be distorted or destroyed in the presence of 2. Place specimen in tea strainer that is suspended over
a super saturated salt solution. funnel.
Step by Step: Fecal Sedimentation
A B C
Test2 (Figure 7)
1. Mix 2 g of feces with water in a cup
(A); remove debris by straining mixture
through gauze or cheesecloth into a
centrifuge-safe tube (B and C).
2. Balance tube and centrifuge at 1500
rpm for 5 minutes (D).
3. Pour off liquid without disturbing the
sediment.
4. Pipette a small amount from the
top layer of sediment (E) onto a
microscope slide; apply a cover slip for viewing. if D E
sediment is too dense and visibility is obscured, dilute
with a drop of water before examining.
Notes:
• If protozoan cysts are suspected, dilute sediment with
Lugol’s iodine for easier viewing.
• If protozoan trophozoites are suspected, evaluate a smear
of the sediment prior to adding Lugol’s iodine, as iodine kills
living trophozoites, impeding visible motility.
July/August 2013 Today’s Veterinary Practice 23
| Today’s Technician
3. Clamp rubber tube closed (tube is attached to end of
funnel). TABLe 4. Common Lungworm Species
4. Pour lukewarm water into the funnel, covering cheese- Seen in domestic Animals
cloth packet.
Lungworm Species Domestic Animal Host
5. Allow specimen to sit undisturbed overnight.
6. The next day, slowly relax clamp to collect a drop of Aelurostrongylus abstrusus cats
fluid on a microscope slide. Add 1 drop of Lugol’s iodine Capillaria aerophila cats
to stain larvae and view (10×) with cover slip.
7. Alternatively: Release 1 to 2 drops of fluid into small test Dictyocaulus arnfieldi donkeys and horses
tube. Add 1 drop of Lugol’s iodine, tap mixture, place Dictyocaulus eckert deer
drop on slide and, with aid of cover slip, view at 10×.
Dictyocaulus viviparus cattle
FECAL CULTURE Metastrongylus apri Pigs
Culturing fecal material is a useful technique when eggs Muellerius capillaris sheep and goats
or cysts cannot be properly distinguished or identified.
Incubation of the culture at room temperature encourages Oslerus osleri dogs
larval hatching and allows for easy identification.2 Protostrongylus rufescens sheep and goats
STAINED FECAL SMEAR
As a confirmatory method of identifying protozoan tro- (to the side) before placing the cover slip on the smear.
phozoites, the fecal smear may be stained. The stain allows 3. Examine the slide using 10x and 40x (do not use oil
identification of oocysts and trophozoites by highlighting immersion). Trophozoites are motile when unstained.
protozoan internal structures. 4. Stain the smear by applying Lugol’s iodine (add 1 drop
Since the iodine stain will kill trophozoites, it is prudent to smear), which will stain the organism’s internal struc-
to examine the fecal smear for motile organisms prior to tures. The trophozoites will no longer be moving after
staining; the motility of trophozoites is what renders them iodine application.
easily identifiable. Nonmoving organisms may be difficult to
identify within the stained smear.1,2 BLOOD ANALYSIS
Heartworms
Step by Step: Stained Fecal Smear1,2 Diagnosis of heartworms is both simple and complex, and
1. Mix a small amount of feces with saline on a microscope dominates veterinary parasitological research. A major focus
slide, creating a thin layer. of research involves identification of this parasite as early as
2. Using a cover slip, move any fecal material out of the way possible postinfection in order to facilitate timely treatment.
Step by Step: Fecal Culture2
Nematode Larvae
1. Place 20 to 30 g of fresh feces in a glass jar. Break up feces and moisten with water; do not add enough water to
make the solution soupy.
2. store away from direct sunlight at room temperature for 7 days. observe daily for condensation inside jar. if
there is no condensation, mist contents lightly to
maintain a moist environment. A B
3. on day 7, collect drops of moisture with small brush or
inoculation loop and transfer them to microscope slide.
4. stain the slide with iodine and kill larvae by passing
slide over Bunsen burner. Killed larvae extend their
bodies, making identification easier.
Coccidial Oocysts (Figure 8)
1. Mix 20 to 30 g of fresh feces with 60 mL of 2.5%
potassium dichromate (A and B).
2. Pour mixture into petri dish (C), cover with lid, and incu-
C D
bate at room temperature for 3 to 5 days. aerate each
day by gently swirling contents with lid removed (D).
3. on day 5, use fecal sedimentation to evaluate the
sample.
4. Follow up by processing fecal sediment with
centrifugal fecal flotation; then examine microscopically
for oocysts.
24 Today’s Veterinary Practice July/August 2013
diagnosis of internal Parasites
Today’s Technician |
Protozoan Parasites buffy coat and plasma for microfilariae movement.
Other parasites may infect various blood cells. 4. If desired, break tube at the buffy coat layer and tap
For instance, Hepatozoon is transmitted by ingestion of plasma onto a slide. Add a drop of methylene blue
infected ticks, most notably Amblyomma maculatum and stain, a drop of physiological saline, and cover slip.
Rhipicephalus sanguineus. It should be noted that H ameri- Observe under 10x objective, with low to medium
canum occurs only in North America and is carried by A light, and scan for microfilariae.
maculatum. Hepatozoon presents on the surface of polymor-
phonuclear leukocytes as well as in skeletal muscle.9 PROTOZOA IDENTIFICATION IN BLOOD
Other protozoan parasites, such as Babesia canis, are Diagnosis of unicellular parasites is easily accomplished
transmitted through the bite of the tick Rhipicephalus san- in a clinical setting by examination of a stained blood
guineus. B canis presents within canine erythrocytes. smear. The best samples to evaluate are those from ani-
mals with an acute infection. A more definitive diagnosis
HEARTWORM IDENTIFICATION or confirmatory testing (such as in the case of Babesia)
The 3 manual methods routinely recognized for immediate may be achieved through diagnostic serology as well as
detection of microfilariae are: utilization of diagnostic laboratory services.
• Direct blood examination (examining a drop of blood Staining may be accomplished using 1 of the following
on a slide) stains:
• Modified Knott’s test (examination of centrifuged blood • Giemsa
sediment) • Quik-Dip Stain (mercedesmedical.com)
• Examination via concentrated filter test (Figure 9). • Dip-Quick Stain (jorvet.com).
The buffy coat method isolates motile microfilariae The gold standard for blood protozoan identification
within the buffy coat layer, which is seen in centrifuged is Giemsa stain, but the quick-dip, 3-step systems also
hematocrit tubes of whole blood, located between the red yield satisfactory results, of which several varieties are
blood cell layer and plasma. This method, while considered commonly used in clinical practice.9
fast, requires more prep time than the methods mentioned
above. It should not be relied upon for definitive diagnosis Step by Step: Giemsa Staining of Blood Film2
because microfilaria may be difficult to see. 1. Air dry a prepared blood film.
Use of enzyme-linked immunosorbent assay (ELISA) 2. Fix in methanol for 5 minutes and air dry again.
testing methods, such as the SNAP Heartworm Test or SNAP 3. Cover with Giemsa stain (1:20 Giemsa:distilled water
4Dx Plus Test (idexx.com), are also useful for in-clinic detec- ratio).
tion of either antigen or antibody in patients with or without 4. Allow to stand for 30 minutes undisturbed.
clinical signs of heartworm disease but no evidence of circu- 5. Gently rinse slide with tap water.
lating microfilaria detected through manual examination of 6. Air dry and view.
blood (due to lack of reproducing females at time of testing). Note: Parasite cytoplasm will stain blue while the nucle-
Testing for antigens produced by parasites remains the us will stain pink/magenta.
most widely used method of detection with regard to heart-
worm disease as it is quick and economically feasible. It ANALYSIS OF URINE
should be noted that more than 1 method is often used to There are several parasites restricted to the urinary
confirm results, whether negative or positive. system, such as the giant kidney worm (Dioctophyma
renale) and bladder worm (Pearsonema plica). The
Step by Step: Buffy Coat Method9 presence of these parasites is most easily confirmed by
1. Prepare a hematocrit tube using whole blood. Be sure to identifying the presence of ova in urine.
seal one end of the tube with sealant clay. Ova may be identified by examining urine sediment
2. Process the tube in a microhematocrit centrifuge at 1500 samples collected through cystocentesis. To avoid con-
rpm for 5 minutes. tamination, cystocentesis is preferred over clean catch,
3. Place centrifuged tube on microscope stage and, using 4x voided specimens. Prior to testing, sample integrity
objective with low light, examine the area between the should be guarded to avoid outside contamination, such
as contact with insects, pollen, or other environmental
debris.2,9
A B
Step by Step: Urine Sedimentation2
1. Place 5 to 10 mL of urine in a conical tube; centrifuge
at 1500 to 2000 rpm for 5 minutes.
2. Decant supernatant; examine the approximately 0.5
mL of sediment left in the tip of the tube.
3. Re-suspend by stirring with a wooden stick or pipette
and transfer a drop to a microscope slide.
Figure 9. dirofilaria immitus microfilariae identified by concen- 4. Add a cover slip and examine the slide for ova.
trated filtering technique with staining at 10× (A) and 40× (B). Note: I have had some success staining the sediment
with Sedi-Stain (bd.com) prior to examination.
July/August 2013 Today’s Veterinary Practice 25
| Today’s Technician | ViTaL VaccinaTion seRies: Fiss
IN SUMMARY (continued from page 57)
• Identification of some parasites requires aseptically col-
for a causal relation between vaccination and fibrosarcoma
lected specimens. tumorigenesis in cats. JAVMA 1993; 203(3):396-405.
• Specimen integrity must be preserved during collec- 7. Gaskell r, Gettinby G, Graham, Skilton d. veterinary Products
tion, transportation, and testing. Committee (vPC) working Group on Feline and Canine
vaccination. department for environment, Food & rural Affairs,
• An up-to-date health history in conjunction with labo- London, May 2001.
ratory testing will allow the clinician to make a more 8. vaccine-Associated Feline Sarcoma Task Force: roundtable
accurate diagnosis. discussion. The current understanding and management of
vaccine-associated sarcomas in cats. JAVMA 2009; 234(3):376-
• Laboratory testing should be conducted using estab- 378.
lished protocols in order to minimize human error. n 9. Shaw SC, Kent MS, Gordon iK, et al. Temporal changes in
characteristics of injection-site sarcomas in cats: 392 cases
eLisa = enzyme-linked immunosorbent assay; Gi = (1990-2006). JAVMA 2009; 234:376-380.
10. Srivastav A, Kass PH, McGill Ld, et al. Comparative vaccine-
gastrointestinal; sG = specific gravity specific and other injectable-specific risks of injection-site
sarcomas in cats. JAVMA 2012; 241(5):595-602.
References 11. Kass PH, Spangler wL, Hendrick MJ, et al. Multicenter case-
1. Zajac A, Conboy G. Fecal examination for the diagnosis of parasitism. control study of risk factors associated with development of
Veterinary Clinical Parasitology, 8th ed. Ames, iA: wiley-Blackwell, 2012, vaccine-associated sarcomas in cats. JAVMA 2003; 223:1283-
pp 4-9, 12-14. 1292.
2. Hendrix C, robinson e. Common laboratory procedures for diagnosing
12. Kass PH, Barnes wG Jr, Spangler wL, et al. epidemiologic
parasitism. Diagnostic Parasitology for Veterinary Technicians, 4th ed. St.
evidence for a causal relation between vaccination and
Louis: elsevier, 2012, pp 316-334, 336.
3. Blagburn BL, Butler JM. Optimize intestinal parasite detection with
fibrosarcoma tumorigenesis in cats. JAVMA 1993; 203:396-405.
centrifugal fecal flotation. Vet Med 2006; 101(7):455-464. 13. Hendrick M. Historical review and current knowledge of risk
4. dryden Mw, Payne PA, ridley r, et al. Comparison of common fecal factors involved in feline vaccine-associated sarcomas. JAVMA
flotation techniques for the recovery of parasite eggs and oocysts. Vet 1998; 213:1422-1423.
Ther 2005; 6:15-28. 14. Lester S, Clemett T, Burt A. vaccine site-associated sarcomas
5. dryden Mw, Payne PA, Smith v. Accurate diagnosis of Giardia spp and in cats: Clinical experience and a laboratory review (1982-1993).
proper fecal examination procedures. Vet Ther 2006; 7:4-14. JAAHA 1996; 32:91-95.
6. Zajac A, Johnson J, King S. evaluation of the importance of centrifugation 15. Macy d, Hendrick M. The potential role of inflammation in the
as a component of zinc sulfate fecal flotation examinations. JAAHA 2002; development of postvaccinal sarcomas in cats. Vet Clin North Am
38:221-224. Small Anim Pract 1996; 26:103-109.
7. Overview of lungworm infection. Merck Veterinary Manual. Assessed 16. Gobar G, Kass P. world wide web-based survey of vaccination
on July 11, 2013, and available at: merckmanuals.com/vet/respiratory_ practices, postvaccinal reactions, and vaccine site-associated
system/lungworm_infection/overview_of_lungworm_infection. sarcomas in cats. JAVMA 2002; 220:1477-1482.
html?qt=lungworms in dogs&alt=sh. 17. richards Jr, elston TH, Ford rB, et al. The 2006 American
8. Bowman d. Georgi’s Parasitology for Veterinarians, 9th ed. St. Louis: Association of Feline Practitioners Feline vaccine Advisory Panel
Saunders-elsevier, 2009, pp 297-298.
report. JAVMA 2006; 229(9):1405-1441.
9. Zajac A, Conboy G. detection of parasites in the blood. Veterinary Clinical
18. Spickler Ar, roth JA. Adjuvants in veterinary vaccines: Modes of
Parasitology, 8th ed. Ames, iA: wiley-Blackwell, 2012, pp 185-192.
action and adverse effects. J Vet Intern Med 2003; 17(3):273-281.
Suggested Reading 19. wilcock B, wilcock A, Bottoms K. Feline postvaccinal sarcoma:
Payne PA, dryden Mw. Accurate evaluation of fecal samples critical to patient. 20 years later. Can Vet J 2012; 53:430-434.
DVM Best Practices 2003; Mar:8-11.
Richard B. Ford, DVM,
MS, Diplomate ACVIM &
Oreta M. Samples, RVT, ACVPM (Hon), is Emeritus
MPH, DHSc, is the lead Professor of Medicine
veterinary technician and at North Carolina State
adjunct instructor at Fort University’s College of
Valley State University in Veterinary Medicine.
Fort Valley, Georgia. She He is a retired Brigadier
also is an adjunct instruc- General from the USAF
tor in epidemiology at Reserve, where he was assigned to the Office
Kaplan University, which of the Surgeon General at the Pentagon. Dr.
has several campuses Ford is also a past president of the NAVC
throughout the U.S., and an adjunct assistant Conference and continues his role as a
professor at St. Francis University in Loretto, member of the scientific program commit-
Pennsylvania. Her research interests include vet- tee. His clinical interests are in the field of
erinary parasitology and clinical pathology. Ms. companion animal infectious disease; he is a
Samples is a founding member of the Academy of prolific author and serves on both the AAHA
Veterinary Clinical Pathology Technicians and an Canine Vaccination Task Force and AAFP
editorial board member for NAVTA. She received Feline Vaccination Advisory Panel. Dr. Ford
her degrees in veterinary technology from Fort received his DVM from Ohio State University
Valley State University and her MS in public health and completed an internal medicine residency
and Doctorate in health sciences from Nova at Michigan State University. He held a previ-
Southeastern University in Fort Lauderdale, Florida. ous faculty position at Purdue University.
26 Today’s Veterinary Practice July/August 2013
You might also like
- Food Microbiology Practical Manual 2020Document67 pagesFood Microbiology Practical Manual 2020jake100% (1)
- ParasitologyDocument18 pagesParasitologyLudwig359100% (2)
- Experiment 1 (Introduction To Microscopy) PDFDocument3 pagesExperiment 1 (Introduction To Microscopy) PDFAisyahNo ratings yet
- SOP For Parasitology Sample Collection and ExaminationDocument11 pagesSOP For Parasitology Sample Collection and ExaminationDr.Kedar Karki ,M.V.Sc.Preventive Vet.Medicine CLSU Philippines88% (8)
- TrematodesDocument9 pagesTrematodesLewis P. SanchezNo ratings yet
- L3 - Microscopic Examinations of Stool PDFDocument5 pagesL3 - Microscopic Examinations of Stool PDFMarianneNo ratings yet
- Platyhelminthes NotesDocument55 pagesPlatyhelminthes Notesapi-375285021No ratings yet
- Parasitology Metazoa: ProtozoaDocument7 pagesParasitology Metazoa: ProtozoaTrishaNo ratings yet
- Phylum Platyhelminthes (Flatworms) : Important InformationDocument26 pagesPhylum Platyhelminthes (Flatworms) : Important InformationYoussef EmadNo ratings yet
- Para Lec Lesson2Document9 pagesPara Lec Lesson2Tolentino, Edron E.No ratings yet
- Parasitology: ProtozoaDocument11 pagesParasitology: ProtozoaJoan BabieraNo ratings yet
- TrematodesDocument9 pagesTrematodesRenien Khim BahayaNo ratings yet
- Class Digenea (Trematoda) - The FlukesDocument10 pagesClass Digenea (Trematoda) - The FlukesAmr EldemardashNo ratings yet
- Pudney 1995 PDFDocument39 pagesPudney 1995 PDFKei MlNo ratings yet
- Different Groups of HelminthsDocument6 pagesDifferent Groups of HelminthsST - Roselyn BellezaNo ratings yet
- Schistosomes and Paragonimus Final - GodoyDocument5 pagesSchistosomes and Paragonimus Final - GodoyDanielle Justine GodoyNo ratings yet
- TrematodesDocument75 pagesTrematodesHann SantiagoNo ratings yet
- Phylum Platyheminthes TrematodesDocument12 pagesPhylum Platyheminthes TrematodesRona SalandoNo ratings yet
- Lecture 3 para Summer 2023-1Document47 pagesLecture 3 para Summer 2023-1محمود سليمانNo ratings yet
- In Vitro Fertilization Ivf 2021Document19 pagesIn Vitro Fertilization Ivf 2021Ahmed AnwarNo ratings yet
- Session 4 Diagnostic Parasitology TechniquesDocument46 pagesSession 4 Diagnostic Parasitology Techniquesannelle0219No ratings yet
- REMETIO - Activity 13 - Urogenital System in AnimalsDocument12 pagesREMETIO - Activity 13 - Urogenital System in AnimalsAnonymous 09No ratings yet
- Introduction To CestodesDocument7 pagesIntroduction To CestodesChristine BuenNo ratings yet
- Parasitology and Clinical MicrosDocument16 pagesParasitology and Clinical MicrosErvette May Niño MahinayNo ratings yet
- Fish Reproduction: 1 AnatomyDocument11 pagesFish Reproduction: 1 Anatomysksingl350No ratings yet
- Introduction To Parasitology PDFDocument17 pagesIntroduction To Parasitology PDFArianna Aparte AvenidoNo ratings yet
- Paralec NotesDocument5 pagesParalec NotesmikaNo ratings yet
- Trematodes General CharacteristicsDocument17 pagesTrematodes General CharacteristicsFreyja PaddamNo ratings yet
- ProtozoaDocument25 pagesProtozoanupur.kmcNo ratings yet
- Tabular Parasitology MICROPARADocument19 pagesTabular Parasitology MICROPARAJerlyn FranciscoNo ratings yet
- I Aim of The ManualDocument42 pagesI Aim of The ManualJOSEPH NDERITUNo ratings yet
- Parasite Lab 5 Helminths PDF (M)Document18 pagesParasite Lab 5 Helminths PDF (M)Ali RonaldoNo ratings yet
- Cestode SDocument79 pagesCestode SVincent Manganaan67% (3)
- Cestodes: 1. Taenia Solium (Pork Tapeworm) & Taenia Saginata (Beef Tapeworm)Document18 pagesCestodes: 1. Taenia Solium (Pork Tapeworm) & Taenia Saginata (Beef Tapeworm)Genevee Ryeleen DelfinNo ratings yet
- Animal Diversity: Zarah Alaska-VillalonDocument68 pagesAnimal Diversity: Zarah Alaska-VillalonGabrielle Salamanca CastuloNo ratings yet
- Classification of HelminthisDocument20 pagesClassification of Helminthisعبدالرحمن عابدNo ratings yet
- Parasitology (Lect #3) TransDocument6 pagesParasitology (Lect #3) TransSherlyn Giban InditaNo ratings yet
- Zoology CH 8 Animal Like Pro TistsDocument103 pagesZoology CH 8 Animal Like Pro TistsTHUNDERCLASH_SILVEREYES139100% (3)
- TrematodesDocument9 pagesTrematodesJoseph PerezNo ratings yet
- PMLS 1ST SEMESTER PREFINAL NOTESDocument14 pagesPMLS 1ST SEMESTER PREFINAL NOTESEben Alameda-PalapuzNo ratings yet
- CESTODE - PPT MajorDocument92 pagesCESTODE - PPT MajorJOSEPH NDERITU100% (2)
- Parasitology: The Supervision of SubjectDocument5 pagesParasitology: The Supervision of Subjectهاشم جليلNo ratings yet
- Infection Fungi Cancer Blood: 1.fecal Analysis Is A Noninvasive Laboratory Test Useful in Identifying Disorders ofDocument4 pagesInfection Fungi Cancer Blood: 1.fecal Analysis Is A Noninvasive Laboratory Test Useful in Identifying Disorders ofDonna IlarNo ratings yet
- Trem-Ing pptIIDocument61 pagesTrem-Ing pptIITutde SedanaNo ratings yet
- OogenesisDocument23 pagesOogenesisFeri ArdiansyahNo ratings yet
- Lab 3 Phage Titration 2014Document6 pagesLab 3 Phage Titration 2014hiteshNo ratings yet
- Animal EvolutionDocument6 pagesAnimal EvolutionYsabella PolanaNo ratings yet
- The Intestinal ProtozoaDocument15 pagesThe Intestinal ProtozoaKHURT MICHAEL ANGELO TIUNo ratings yet
- ParasitologyDocument4 pagesParasitologyJovanni andesNo ratings yet
- GlossaryDocument37 pagesGlossaryTiff UyNo ratings yet
- Cestodes: Diphyllobothrium Latum, Broad or FishDocument2 pagesCestodes: Diphyllobothrium Latum, Broad or FishFrance Louie JutizNo ratings yet
- Virology and Immunology PracticalDocument6 pagesVirology and Immunology PracticallivehologramNo ratings yet
- Title ReportDocument2 pagesTitle ReportBoaz OngaroNo ratings yet
- Ece30005 3023Document8 pagesEce30005 3023Diary ThalitaNo ratings yet
- Nematodes: Ascaris Lumbricoides Trichuris Trichiura Enterobius VermicularisDocument10 pagesNematodes: Ascaris Lumbricoides Trichuris Trichiura Enterobius VermicularisAbby SiervoNo ratings yet
- Structure and Function of Protozoa: Phagocytic CapacityDocument1 pageStructure and Function of Protozoa: Phagocytic Capacityabigail correNo ratings yet
- Para-Transes Midterm Exam - Unit 3Document8 pagesPara-Transes Midterm Exam - Unit 3Aysha AishaNo ratings yet
- A Complete Note On Parasitology For 2nd PDFDocument105 pagesA Complete Note On Parasitology For 2nd PDFMahi SinghNo ratings yet
- Cestodes Trematodes: Agustina Tri Endharti Ssi.,Ph.DDocument42 pagesCestodes Trematodes: Agustina Tri Endharti Ssi.,Ph.DAndre_rarungNo ratings yet
- PARASITOLOGYDocument13 pagesPARASITOLOGYJoel CabañasNo ratings yet
- 1st Lecture - Trematodes - Clinical ParasitologyDocument38 pages1st Lecture - Trematodes - Clinical ParasitologyAhmed MoghazyNo ratings yet
- Camp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.From EverandCamp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.No ratings yet
- Pigeons - A Collection of Articles on the Origins, Varieties and Methods of Pigeon KeepingFrom EverandPigeons - A Collection of Articles on the Origins, Varieties and Methods of Pigeon KeepingNo ratings yet
- Daftar Pendaftar 05-08-2020Document6 pagesDaftar Pendaftar 05-08-2020MonicaCeciliaNo ratings yet
- Minggu 3 Suplemen 2 Lung PatternDocument8 pagesMinggu 3 Suplemen 2 Lung PatternMonicaCeciliaNo ratings yet
- IB Domba-Ayam-Covid 19 PDFDocument31 pagesIB Domba-Ayam-Covid 19 PDFMonicaCeciliaNo ratings yet
- Monica Cecilia - B0901201036 - Resep Pyoderma PDFDocument2 pagesMonica Cecilia - B0901201036 - Resep Pyoderma PDFMonicaCeciliaNo ratings yet
- Ipi 82609Document9 pagesIpi 82609Ieta KeyNo ratings yet
- Jurnal Riset Veteriner IndonesiaDocument4 pagesJurnal Riset Veteriner IndonesiaMonicaCeciliaNo ratings yet
- Jurnal Riset Veteriner IndonesiaDocument4 pagesJurnal Riset Veteriner IndonesiaMonicaCeciliaNo ratings yet
- Ethylene 20 Glycol 20 OverviewDocument11 pagesEthylene 20 Glycol 20 OverviewMonicaCeciliaNo ratings yet
- Blood CollectionDocument6 pagesBlood CollectionSol Kizziah MeiNo ratings yet
- Lab Exercise 1 MICROSDocument9 pagesLab Exercise 1 MICROSCrizzajen IsipNo ratings yet
- LEUKOcyte Counting LabDocument13 pagesLEUKOcyte Counting Labsyed nomanshahNo ratings yet
- Lab Report Bio122Document19 pagesLab Report Bio122Amran NazriNo ratings yet
- Pap StainingDocument2 pagesPap StainingDrManish KumarNo ratings yet
- Lab Exercise 2 Microscope Anph111Document5 pagesLab Exercise 2 Microscope Anph111Jhon Leonard FatalloNo ratings yet
- 9700 - MW - Script E - P3Document20 pages9700 - MW - Script E - P3Y. PurwandariNo ratings yet
- Microscope and Basic Plant Microtechnique Activity 2 WorksheetDocument9 pagesMicroscope and Basic Plant Microtechnique Activity 2 WorksheetEPHRAIM JOASH ABEJO GAGANTINGNo ratings yet
- Science 10 Textbook Unit ADocument132 pagesScience 10 Textbook Unit AessamNo ratings yet
- Laboratory Methods - BAM - Yeasts, Molds and MycotoxinsDocument10 pagesLaboratory Methods - BAM - Yeasts, Molds and MycotoxinsBhey NicoleNo ratings yet
- Science 7 Compendium 2nd QTR With CoverDocument66 pagesScience 7 Compendium 2nd QTR With CoverLyra Mae De BotonNo ratings yet
- Labmanual ExperimentsinsixthformbiologyDocument93 pagesLabmanual ExperimentsinsixthformbiologyturaganivaluaniNo ratings yet
- Biology 3rd Lab Report (Animal Cell)Document4 pagesBiology 3rd Lab Report (Animal Cell)Johanna Haludilu67% (3)
- Pub - The Rat Brain in Stereotaxic Coordinates The New CDocument210 pagesPub - The Rat Brain in Stereotaxic Coordinates The New CAttila Szakacs100% (1)
- LAB - Plant Vs Animal CellsDocument4 pagesLAB - Plant Vs Animal CellsAljeanNo ratings yet
- Lab Report 2 - CytologyDocument7 pagesLab Report 2 - CytologyDa Nie L100% (1)
- Concepts of Biology - Lab ManualDocument43 pagesConcepts of Biology - Lab ManualUMMU MARDHIAH ABDUL HALIMNo ratings yet
- Biaka University Institution of Buea (Buib) : "The Audacity To Be Different"Document24 pagesBiaka University Institution of Buea (Buib) : "The Audacity To Be Different"BIKOBO DESTAINGNo ratings yet
- DOH Equipment Clinical LabDocument6 pagesDOH Equipment Clinical Labal gulNo ratings yet
- 1a.biological TechniquesDocument23 pages1a.biological TechniquesPlayersbattle GroundNo ratings yet
- Lab Report For Mic461Document3 pagesLab Report For Mic461Puteri NursyafiqahNo ratings yet
- Laboratory Activity Number 3Document31 pagesLaboratory Activity Number 3Poala Hannah MateoNo ratings yet
- Experiment 3 Bio300Document5 pagesExperiment 3 Bio300ellymanisNo ratings yet
- Pathology by Dr. (Prof) S.K. Samanta (CFSL) PDFDocument113 pagesPathology by Dr. (Prof) S.K. Samanta (CFSL) PDFNeelotpalNo ratings yet
- Investigating Plant CellsDocument3 pagesInvestigating Plant CellsJun Bryan C. AcobNo ratings yet
- Biology 10-12 Revised Edition 2Document138 pagesBiology 10-12 Revised Edition 2Mwami JayNo ratings yet
- Final Pakage Biology SSC-1 FormatedDocument8 pagesFinal Pakage Biology SSC-1 FormatedNafeesa MaryamNo ratings yet