Professional Documents
Culture Documents
Solutions and Stoichiometry Worksheet (Sept, 17, 2021)
Uploaded by
Ahmed AlmossaweOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solutions and Stoichiometry Worksheet (Sept, 17, 2021)
Uploaded by
Ahmed AlmossaweCopyright:
Available Formats
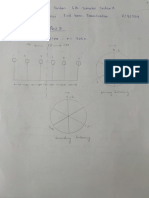
Problem Set A Concentration
Concentration x 732mold
It
I
2omolKOH
2 M 2 omoldm M 2.0
2 OX56.11gmol112.20
9 i mo
3 5mot licks concentration x
ffdi.de 4moldmsl
10 31 5 0.67molkott concentration x 8TT 34moldmkoH
t
Problem Set B Concentration
1 49 0.4225 mol NaOH concentration x 8EE l 69mold
fuk Lof
concentration x 8hn 0.24moldniNHy
Gg
Tgm
gg
molarmass
of
211 1 EEÉ i
8 NHyCl 53.5gmol l Concentration 0.875 dm 0.3 0.875 0 263mot
0.263molF53.5gmolt 14.1gNHy
97 AgNo 169.9 gmot
0.214dm x1000 214.3
08711 V 871
IF 0 07mol Concentration o.og
214.3cm'Ag
Problem Set C Dilutions MV Mav
1 V2 25 125 150cm M Y Mak Mz 11 Mz 4EIst o1zsmoldm
M 0.1s V 12
27 VIdi8 I soon Ma MI Mz off crinoid
3 Y I 50,000cm3sodmsor5xioscm
1 1,1 Vz ve
4J.VM 345cm
1 S V 2s Mz MI Mz IE 2.07moldn
11 CHCOONa 82.04gmol 857 0.8mot Concentration X 8151 16
I.tmoldmcHsCOONa
I3 KOH 56 Igmol 5
9 0.9mot KOH
Concentration0.77 09701 V É 1.2dm3kott
147 X amount of waterto add ml om
concentration 0.3578
Vtx dEsx
21y.IE 1.4dm3
1.41itersofwater
15
Yi v MI v
Problem Set
Fedffind
D Solution Stoichiometry
1 2HCl t Na CO Hao t CO A NaCl
0.54 mol Hd concentration 1 5
95 V 0.36
O 36 dm x 1000 360cm 36om
Cull concentration 0.777 145 3
2 Zn f Cu Cla
O 33 mot X 65.38 21
ZnCla t Cu I 3 IIF o.omoien
Gg2nwillbeused
Barium Nitrate
3 Ba Nos
Ba Nos at Nancy Basoy 2NaNO
JNazSoyconcentIion0.086 ITEM 0.1570.086 0.013Mol Na 504
BalNoi
0.013mA x 11115T Egmol
Ba Nos concentration10.232
kid
V 894mF 0.056dm x 1000 56.0cm3BalN
You might also like
- Refrigeration and Air Conditioning Chapter8Document51 pagesRefrigeration and Air Conditioning Chapter8Richard Weimer100% (2)
- 3 מטלהDocument6 pages3 מטלהניצן גונןNo ratings yet
- Din 3967 1978 Eng PDFDocument24 pagesDin 3967 1978 Eng PDFJosé Francisco Ramos Teixeira100% (2)
- HVOF CoatingDocument3 pagesHVOF CoatingsumohiNo ratings yet
- S 000 5310 001 (Structual)Document33 pagesS 000 5310 001 (Structual)Midhun K ChandraboseNo ratings yet
- Shriram PistonDocument46 pagesShriram Pistondeepak GuptaNo ratings yet
- SAEP-351 Bolted Flange Joint AssemblyDocument12 pagesSAEP-351 Bolted Flange Joint AssemblyBebin Mathew75% (8)
- 3-Vapour Compression SystemsDocument18 pages3-Vapour Compression SystemsUtkarsh SinghNo ratings yet
- 1.1table - Mineral ChemistryDocument3 pages1.1table - Mineral Chemistryjako_kcNo ratings yet
- Practice AnswersDocument11 pagesPractice AnswersibrahimNo ratings yet
- Act. 3 ChemistryDocument2 pagesAct. 3 ChemistryApril Mae BaldozaNo ratings yet
- Chapter 4. NotesDocument11 pagesChapter 4. Notesayshhaa.kNo ratings yet
- General ChemistryDocument1 pageGeneral ChemistryBailey PryorNo ratings yet
- Adobe Scan 01-Jan-2024Document11 pagesAdobe Scan 01-Jan-2024Dhananjay SenNo ratings yet
- Pages de Cambridge IB Chemistry-11Document1 pagePages de Cambridge IB Chemistry-11Tanguy PocquetNo ratings yet
- Adobe Scan Feb 15, 2023Document4 pagesAdobe Scan Feb 15, 2023Versoza AltaNo ratings yet
- Experiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Document6 pagesExperiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Zahra Al-BasriNo ratings yet
- Tugas 1 - Eka Kurniati Kombong Datu - 05201026Document9 pagesTugas 1 - Eka Kurniati Kombong Datu - 05201026시우민SeohyunNo ratings yet
- Fall 2020 CH115 Final Exam ASC Review Annotated Slides - TimeDocument24 pagesFall 2020 CH115 Final Exam ASC Review Annotated Slides - TimeKrumpus H.No ratings yet
- Adobe Scan Sep 30 2022Document1 pageAdobe Scan Sep 30 2022John Rey BalansagNo ratings yet
- Spectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledDocument19 pagesSpectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledM Irfan Khan100% (1)
- Curva Estandar MineralesDocument2 pagesCurva Estandar MineralesMichel Jer AbzNo ratings yet
- Act 8 1 Mole Practice KEYevenDocument3 pagesAct 8 1 Mole Practice KEYevenHassan warriachNo ratings yet
- Results and Calculation: 3 - (Aq) - (Aq) + (Aq) 3 - (Aq) 2 (Aq)Document20 pagesResults and Calculation: 3 - (Aq) - (Aq) + (Aq) 3 - (Aq) 2 (Aq)myzna_husna_90788547No ratings yet
- Tugas 1 40040117060067 SyadilalutfimDocument6 pagesTugas 1 40040117060067 SyadilalutfimSyadila LutfiNo ratings yet
- Adobe Scan Jan 19 2023 PDFDocument5 pagesAdobe Scan Jan 19 2023 PDFJohn Rey BalansagNo ratings yet
- 112.1 Exercise 9Document3 pages112.1 Exercise 9Julie Ann FelicesNo ratings yet
- Note 18 Oct 2023Document5 pagesNote 18 Oct 2023Elif KucukdagNo ratings yet
- New Tutorial 8 With SolutionDocument5 pagesNew Tutorial 8 With SolutionNaveed AhmadNo ratings yet
- 18UME006 DOM End Term May 07, 2021Document7 pages18UME006 DOM End Term May 07, 2021nerd devilNo ratings yet
- Experiment 5 - Data TreatmentDocument6 pagesExperiment 5 - Data TreatmentShawn Ann SilanNo ratings yet
- General Chemistry Module 4 OutputDocument3 pagesGeneral Chemistry Module 4 OutputRyah Lyn RevaleNo ratings yet
- Lab 7Document3 pagesLab 7api-341253869No ratings yet
- (18998526 - Mineralogia) Mineralogy and Petrology of Two Ordinary Chondrites and Their Correlation With Other MeteoritesDocument9 pages(18998526 - Mineralogia) Mineralogy and Petrology of Two Ordinary Chondrites and Their Correlation With Other MeteoritesMauro MarafonNo ratings yet
- CH 4 Test Review sheet-KEYDocument4 pagesCH 4 Test Review sheet-KEYNaomi HeywardNo ratings yet
- Mass and Molar MassDocument3 pagesMass and Molar MassColleen Dela CruzNo ratings yet
- Ex 3Document2 pagesEx 3Parth KadamNo ratings yet
- Homework Week 4 MinhDocument10 pagesHomework Week 4 MinhSiddhant BhardwajNo ratings yet
- ANACHEMDocument8 pagesANACHEMVince Andrew BombitaNo ratings yet
- AFT Assignment 2Document3 pagesAFT Assignment 2Shakthi Prasad PNo ratings yet
- שאלה קיר כובד-1Document6 pagesשאלה קיר כובד-1foaad.zbedat1No ratings yet
- Chapter2 Answers PDFDocument12 pagesChapter2 Answers PDFLalu SuhaimiNo ratings yet
- Kurva Baku DistilasiDocument3 pagesKurva Baku DistilasiNur KholisaNo ratings yet
- Jawaban SlideDocument3 pagesJawaban SlideCitra SalbellaNo ratings yet
- Latihan Soal Bab 1 Viskositas Dan Mekanisme Perpindahan Momentum 1. Calculation of Viscosities of Gas Mixtures at Low DensityDocument27 pagesLatihan Soal Bab 1 Viskositas Dan Mekanisme Perpindahan Momentum 1. Calculation of Viscosities of Gas Mixtures at Low DensityFettyNo ratings yet
- LHCH 1 AnDocument3 pagesLHCH 1 Annishadshivam74354No ratings yet
- BET SolvedProblemsDocument5 pagesBET SolvedProblemsa6167566No ratings yet
- Balance General Balance H2 : Resolver (1) y (2) SimultaneamenteDocument10 pagesBalance General Balance H2 : Resolver (1) y (2) SimultaneamenteDanitza Lizet Zavala ReynaNo ratings yet
- KS13 #3 PDFDocument7 pagesKS13 #3 PDFSwastik TripathiNo ratings yet
- Informe Industrial PrimeroDocument5 pagesInforme Industrial PrimeroKatheryn RamirezNo ratings yet
- Tugas 1 Transformasi Fasa MaterialDocument6 pagesTugas 1 Transformasi Fasa MaterialIkhwan Nur Rahman ArNo ratings yet
- Avogadno U M B E Na: Mole Comce PT UmbeyDocument2 pagesAvogadno U M B E Na: Mole Comce PT UmbeyBhäùtík SåvälìyâNo ratings yet
- Contoh Soal Balok KolomDocument8 pagesContoh Soal Balok KolomDZAKI MuflihNo ratings yet
- Exam 1 Reminder: - Closed Book, Closed Notes and HW - Calculator Required - 2 Hour Time Limit - Thursday Through MondayDocument7 pagesExam 1 Reminder: - Closed Book, Closed Notes and HW - Calculator Required - 2 Hour Time Limit - Thursday Through MondayIllidari OlimpocronosNo ratings yet
- 4.13 ReviewDocument3 pages4.13 ReviewVansh PatelNo ratings yet
- Empirical Formula HardnessDocument11 pagesEmpirical Formula HardnessMatteo CarusoNo ratings yet
- Solutions Set12Document18 pagesSolutions Set12Frank CañasNo ratings yet
- Tutorial 1 and SolutionsDocument9 pagesTutorial 1 and Solutionshoboslayer97No ratings yet
- Positively Charged Gold Nanoparticles FoDocument26 pagesPositively Charged Gold Nanoparticles FoMuhammad ZeeshanNo ratings yet
- Apc TutDocument74 pagesApc TutkunamallarajendraprasadNo ratings yet
- Kurva Standar Total Fenol (MG/DL) : Konsentrasi Absorbansi 0.2 0.231 0.4 0.428 0.6 0.652 0.8 0.865 1 1.045Document3 pagesKurva Standar Total Fenol (MG/DL) : Konsentrasi Absorbansi 0.2 0.231 0.4 0.428 0.6 0.652 0.8 0.865 1 1.045Fuad KoasNo ratings yet
- Experiment Eight: Spectrophotometric Analysis of A Complex MixtureDocument12 pagesExperiment Eight: Spectrophotometric Analysis of A Complex MixtureMark AwNo ratings yet
- Partition and Bulk Dis. Coeff. Igneous Petrology Practical (GLC514) Laboratory ManualDocument34 pagesPartition and Bulk Dis. Coeff. Igneous Petrology Practical (GLC514) Laboratory Manualfigesab482No ratings yet
- Penentuan Uji Kelarutan KTI ParacetamolDocument6 pagesPenentuan Uji Kelarutan KTI ParacetamolMinul1412No ratings yet
- Recipe For B&D (Broughton and Dilworth 1971) Nutrient Solution For Growing LegumesDocument2 pagesRecipe For B&D (Broughton and Dilworth 1971) Nutrient Solution For Growing LegumesKalpanaNo ratings yet
- Changing From Element WT% To Oxide WT%Document11 pagesChanging From Element WT% To Oxide WT%Reuben De BruynNo ratings yet
- Formularium Obat Generik BPJSDocument12 pagesFormularium Obat Generik BPJSNoraPutriNo ratings yet
- Captek™ - A New Capillary Casting Technology For Ceramometal RestorationsDocument12 pagesCaptek™ - A New Capillary Casting Technology For Ceramometal RestorationsRiya KvNo ratings yet
- A Novel Fabrication Method For Micro Optical Waveguide Mold Based On Y-Cutting TechnologyDocument3 pagesA Novel Fabrication Method For Micro Optical Waveguide Mold Based On Y-Cutting TechnologyRizky FirmansyahNo ratings yet
- Investigatory Project Proposal in Integrated Science 1Document12 pagesInvestigatory Project Proposal in Integrated Science 1safyr94% (35)
- Biology QuestionsDocument24 pagesBiology QuestionssophiegarciaNo ratings yet
- Sri Chandra Sekharendra Saraswathi Vishwa MahavidyalayaDocument132 pagesSri Chandra Sekharendra Saraswathi Vishwa Mahavidyalayabollina lakshmi bhargaviNo ratings yet
- Class - XiDocument35 pagesClass - XiKirti PathakNo ratings yet
- MV - Cosmic Tiger FORMAT RENDALDocument11 pagesMV - Cosmic Tiger FORMAT RENDALHendra MurdiyonoNo ratings yet
- Albertisia Papuana BeccDocument5 pagesAlbertisia Papuana BeccEva MayasariNo ratings yet
- Oct.4 8 Chem 1 WorksheetDocument3 pagesOct.4 8 Chem 1 WorksheetXander Christian RaymundoNo ratings yet
- Food MCB II NotesDocument73 pagesFood MCB II NotesRichard Simon KisituNo ratings yet
- Asam UratDocument38 pagesAsam UratRai Fit TimikaNo ratings yet
- Revision Notes - Unit 1: ParticlesDocument38 pagesRevision Notes - Unit 1: ParticlesAtom D'ArcangeloNo ratings yet
- SOP Soil Loss On IgnitionDocument2 pagesSOP Soil Loss On Ignitionhui143No ratings yet
- Fracture MechanicsDocument47 pagesFracture MechanicsDEEPAKNo ratings yet
- A Background Study On Water Activated FlashlightDocument5 pagesA Background Study On Water Activated FlashlightAllyzon Acosta20% (5)
- Laticrete Hydroban Tds NFDocument6 pagesLaticrete Hydroban Tds NFAbdul Raheem SyedNo ratings yet
- Merrill CroweDocument7 pagesMerrill CroweAlejandro ClavijoNo ratings yet
- Operator (Physics) - WikipediaDocument9 pagesOperator (Physics) - Wikipediaeimc2No ratings yet
- CH Cooh (A) + CH CH CH CH OH (B) (C) + H O (D) : Ch3Ch2Ch2Cooch2Ch3Document5 pagesCH Cooh (A) + CH CH CH CH OH (B) (C) + H O (D) : Ch3Ch2Ch2Cooch2Ch3wanNo ratings yet
- Gypsum ProductsDocument53 pagesGypsum ProductsKiran KumarNo ratings yet
- Histological Studies On Egg Development in Painted Grasshopper, Poekilocerus PictusDocument6 pagesHistological Studies On Egg Development in Painted Grasshopper, Poekilocerus Pictusmrazivbu23No ratings yet
- Problemset PDFDocument69 pagesProblemset PDFTrương ThiênNo ratings yet