Professional Documents
Culture Documents
Alcohols Esther Thiols
Uploaded by
Aeva CamachoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alcohols Esther Thiols
Uploaded by
Aeva CamachoCopyright:
Available Formats
ALCOHOLS, ESTHER, AND THIOLS:
Alcohols:
Organic compounds that contain a hydroxyl group (-OH)
Classified by the number of carbon atoms directly bonded to the carbon attached to the
hydroxyl group
o Primary alcohol - 1 C atom bonded to C-OH
o Secondary alcohol - 2 C atoms bonded to C-OH
o Tertiary alcohol - 3 C atoms bonded to C-OH
Properties of Alcohols:
Polar molecule
o b/c of ΔEN of C-O bond and O-H bond
Capable of hydrogen bonding
High boiling point and melting point
o Due to strong intermolecular forces and high polarity
Alcohols with short hydrocarbon chains are soluble in water
o Less soluble with longer hydrocarbon chains
Naming Alcohols:
Use the same rules as alkanes, alkenes, and alkynes
Replace the final –e with –ol
o Use di, tri, etc. to indicate the number of –OH
o Use numbers to indicate the carbon the –OH is bonded to
For rings, -OH is on the first carbon
Practice for Alcohols:
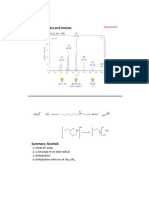
Example:
a) 5,6-diethyl-2,7-dimethyloctane-3,4-diol
b) 4-chloro-5-cyclopentyl-2-hexanol
E T H E R S:
Organic compounds that has an oxygen atom bonded between two carbon atoms
Properties of Ethers:
Slightly higher m.p. and b.p. but lower than alcohols
o C – O bond is polar, but not capable of H-bonding
Useful Solvents
o Can dissolve highly polar substances and Less polar substances
Naming Ethers:
Method #1:
o Find the longest chain
o Name both hydrocarbon chains
o Consider shorter chain to be substituent and end with “oxy” instead of “yl”
Remember to add the C number
o Longer chain is treated as the parent chain
Method #2:
o Name both hydrocarbon chains
Longer chain ends with “ane/ene/yne”
o Add word “ether”
Cyclic ethers are named as oxacycloalkanes with the oxygen being at position 1 of the
chain
Practice for Ethers:
Examples:
a) 2-bromo-4-ethyl-3-(1-methylethoxy)hexane
b) 2-ethyloxacyclohexan-3-ol
T H I O L S:
Organic compounds containing a sulfhydryl group (S-H)
Properties of Thiols:
Strong odors
o Ex. Garlic, skunk smell, sewage
B.p. and m.p. close to alkanes
o b/c nonpolar bonds and same intermolecular forces
Naming Thiols:
Use the same rules as alkanes, alkenes, and alkynes
To the ending (keep the e), add –thiol
o Use di, tri, etc. to indicate the number of –SH
o Use numbers to indicate the carbon the –SH is bonded to
For rings, -SH is on the first carbon
You might also like
- Chm102 Lec - FinalsDocument21 pagesChm102 Lec - FinalsChurva EklavuNo ratings yet
- Alcohols, Phenols and EthersDocument28 pagesAlcohols, Phenols and EthersDnyanesh Shinde100% (1)
- Oxygen Containing CompoundsDocument15 pagesOxygen Containing Compoundsguia macatangayNo ratings yet
- 1.4 Alcohols, Ethers, ThiolsDocument17 pages1.4 Alcohols, Ethers, ThiolsMia PereiraNo ratings yet
- 4 - Nomenclature of Alcohols & EthersDocument8 pages4 - Nomenclature of Alcohols & Ethershazalsakli13No ratings yet
- Unit 11 - Alcohols, Phenols and EthersDocument41 pagesUnit 11 - Alcohols, Phenols and EthersPoovaraahan RaghuveeranNo ratings yet
- Organic Chem U-2 AlcoholDocument33 pagesOrganic Chem U-2 Alcoholsinte beyuNo ratings yet
- Carbon CompoundDocument44 pagesCarbon CompoundriverarafaelljamesNo ratings yet
- Aldehyde and Ketones FDocument70 pagesAldehyde and Ketones Fmichelmanirakiza591No ratings yet
- Chapter 3Document28 pagesChapter 3c4.arsyadNo ratings yet
- Kakhasan Atom Karbon Dan Gugus FungsinyaDocument34 pagesKakhasan Atom Karbon Dan Gugus FungsinyaAllright ShitNo ratings yet
- Unit 14 - Organic ChemistryDocument56 pagesUnit 14 - Organic ChemistryRey GoldNo ratings yet
- 2021 1.5 - 1.7 Double Bonded Functional GroupsDocument27 pages2021 1.5 - 1.7 Double Bonded Functional GroupsMia PereiraNo ratings yet
- Alkohol Dan Eter: Imas Masruroh, M.SiDocument68 pagesAlkohol Dan Eter: Imas Masruroh, M.SiIndah Rizki ManjayantiNo ratings yet
- Gen - Chem Module 3 PDFDocument8 pagesGen - Chem Module 3 PDFWendellNo ratings yet
- Organic Chemistry CurrentDocument48 pagesOrganic Chemistry CurrentBierzo JomarNo ratings yet
- Organic ChemistryDocument55 pagesOrganic ChemistryTechnology Developer ChannelNo ratings yet
- Introduction To Orgnic ChemistryDocument27 pagesIntroduction To Orgnic ChemistryladybugNo ratings yet
- Aldehydes and KetonesDocument7 pagesAldehydes and KetonesAshok PradhanNo ratings yet
- NomenclatureDocument38 pagesNomenclatureMary Darice WongNo ratings yet
- Functional Groups - Organic ChemistryDocument61 pagesFunctional Groups - Organic ChemistryYoAmoNYCNo ratings yet
- Oxygen Containing Organic CompoundsDocument9 pagesOxygen Containing Organic CompoundsmNo ratings yet
- Carbon and Its Compound NotesDocument30 pagesCarbon and Its Compound Noteskrishna industries100% (1)
- Снимок экрана 2021-11-09 в 10.50.11Document38 pagesСнимок экрана 2021-11-09 в 10.50.11Book DragonNo ratings yet
- Lesson 8 Aldehyds, KetonesDocument54 pagesLesson 8 Aldehyds, KetonesIris BallajNo ratings yet
- Organic Chemistry PPT - Ch. 22 - 11th Grade - II TERM - DeC'23Document138 pagesOrganic Chemistry PPT - Ch. 22 - 11th Grade - II TERM - DeC'23Ana Pamela MejiaNo ratings yet
- Introduction To HYDROCARBONDocument12 pagesIntroduction To HYDROCARBONMohamad AzaniNo ratings yet
- Alcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"Document33 pagesAlcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"AmanNo ratings yet
- Carbon and Its CompoundsDocument22 pagesCarbon and Its Compoundsapi-24735088294% (17)
- AlkenesDocument52 pagesAlkeneszaharanuraaNo ratings yet
- Alcohol1 ProjectDocument6 pagesAlcohol1 Projectanchalbaburaj06No ratings yet
- Organic Chemistry Some Basic Principles and TechniquesDocument33 pagesOrganic Chemistry Some Basic Principles and TechniquesAditya Jalal100% (2)
- cHEM 11 uNIT 5 OrgchemDocument68 pagescHEM 11 uNIT 5 OrgchemAce CardenoNo ratings yet
- Brady Ch25-Organic Biochem PDFDocument55 pagesBrady Ch25-Organic Biochem PDFAlams Rizan FirdausNo ratings yet
- The Saturated Hydrocarbons: Alkanes and Cycloalkanes: Contrasts Between Organic and Inorganic MoleculesDocument9 pagesThe Saturated Hydrocarbons: Alkanes and Cycloalkanes: Contrasts Between Organic and Inorganic MoleculesNaveenNo ratings yet
- Chem1-Lec11-Other Organic CompoundsDocument60 pagesChem1-Lec11-Other Organic CompoundsSkud GuillermoNo ratings yet
- Module 4 - Alcohol, Ether and AldehydeDocument62 pagesModule 4 - Alcohol, Ether and AldehydePrincess NavarroNo ratings yet
- Nomenclature - Carbon BondingDocument28 pagesNomenclature - Carbon BondingRobertMurray100% (1)
- Alcohols, Phenols & EthersDocument27 pagesAlcohols, Phenols & Ethershgp9ms5gjcNo ratings yet
- Alcohols, Organic ChemistryDocument32 pagesAlcohols, Organic Chemistryclassy43390% (1)
- Functional G Chem 15th FebDocument64 pagesFunctional G Chem 15th FebAndrew GordonNo ratings yet
- Nomenclature AllDocument83 pagesNomenclature AllLabib HasnainNo ratings yet
- Alcohols: Alcohol, Any of A Class ofDocument3 pagesAlcohols: Alcohol, Any of A Class ofCool for the AnimeNo ratings yet
- Aldehydes and Ketones-DSVOLDocument107 pagesAldehydes and Ketones-DSVOLMERCY ATUYANo ratings yet
- Alchohols, Ethers, PhenolsDocument45 pagesAlchohols, Ethers, PhenolsMohammed JunaidNo ratings yet
- AlcoholsDocument22 pagesAlcoholsSai Sasivardhan GampaNo ratings yet
- Introduction To Organic ChemistryDocument18 pagesIntroduction To Organic ChemistryElmarNo ratings yet
- Functional GroupDocument45 pagesFunctional Groupmonasteriomatthew7No ratings yet
- Class 12 - Alcohols Phenols Ethers-1Document106 pagesClass 12 - Alcohols Phenols Ethers-1Ridhi AgarwalNo ratings yet
- Chapter - 4: Carbon and Its CompoundsDocument22 pagesChapter - 4: Carbon and Its CompoundsshahrukhNo ratings yet
- f322 Mod1Document16 pagesf322 Mod1api-275024237No ratings yet
- Alcohol and HalidesDocument27 pagesAlcohol and HalidesJohn DuranoNo ratings yet
- Alcohol & Phenol: Primary Secondary Tertiary AromaticDocument32 pagesAlcohol & Phenol: Primary Secondary Tertiary AromaticcikguhafidzuddinNo ratings yet
- Organic Compounds and NomenclatureDocument22 pagesOrganic Compounds and NomenclatureNunaNo ratings yet
- Chapter 15 Intro To OrganicDocument8 pagesChapter 15 Intro To OrganicLisa DentonNo ratings yet
- Lab 01 Structure and BondingDocument19 pagesLab 01 Structure and BondingynottripNo ratings yet
- Final Exam 40% Exams 45% Report 5% Homework 10%Document74 pagesFinal Exam 40% Exams 45% Report 5% Homework 10%kaleijaNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- 12 Step Support GroupsDocument26 pages12 Step Support GroupsKim MarquardNo ratings yet
- CHEM 1315 Exam 3 Sp11 v2 Answer KeyDocument8 pagesCHEM 1315 Exam 3 Sp11 v2 Answer KeyAlex BarberieNo ratings yet
- Make Mead Like A Viking: Traditional Techniques For Brewing Natural, Wild-Fermented, Honey-Based Wines and Beers - Jereme ZimmermanDocument5 pagesMake Mead Like A Viking: Traditional Techniques For Brewing Natural, Wild-Fermented, Honey-Based Wines and Beers - Jereme ZimmermanzaladiryNo ratings yet
- Sensory Evaluation of Beverages Containing Alcohol: Standard Guide ForDocument7 pagesSensory Evaluation of Beverages Containing Alcohol: Standard Guide ForEric GozzerNo ratings yet
- L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.1Document21 pagesL.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.1IEyra ShaHeraNo ratings yet
- Borohydride IodineDocument4 pagesBorohydride IodineBandita DattaNo ratings yet
- Performance Chemicals GuideDocument45 pagesPerformance Chemicals Guidetopguitar100% (1)
- 2CDocument28 pages2CAbhinav BhatnagarNo ratings yet
- Carboxylic Acid - SPM ChemistryDocument11 pagesCarboxylic Acid - SPM ChemistryVinnySha SelvarajahNo ratings yet
- Topic 11: Organic Chemistry 11.1 Homologous SeriesDocument8 pagesTopic 11: Organic Chemistry 11.1 Homologous SeriesbnNo ratings yet
- Brand NameDocument4 pagesBrand NameAnjnaKandariNo ratings yet
- KCET Chemistry Analysis and Study PlannerDocument5 pagesKCET Chemistry Analysis and Study PlannerS. FASEEH MNo ratings yet
- Classification Tests For Carboxylic Acid and DerivativesDocument3 pagesClassification Tests For Carboxylic Acid and DerivativesJohn Emmanuel SyNo ratings yet
- RSV 1Document9 pagesRSV 1DEBRAJ SINHA ROYNo ratings yet
- Khodays Since 1906Document15 pagesKhodays Since 1906vikas yadav0% (1)
- Wine and Spirits PDFDocument11 pagesWine and Spirits PDFNavaneetDDeshpandeNo ratings yet
- Silabo - 17201Document7 pagesSilabo - 17201Enrique Rojas BermudoNo ratings yet
- Lecture18 2Document65 pagesLecture18 2Betty WeissNo ratings yet
- Mcqs For Xii - Chemistry: Page 1 of 24Document24 pagesMcqs For Xii - Chemistry: Page 1 of 24MUKHTIAR HassanNo ratings yet
- Alcohol: This Article Is About The Class of Chemical Compounds. For Found In, See - For Other Uses, SeeDocument4 pagesAlcohol: This Article Is About The Class of Chemical Compounds. For Found In, See - For Other Uses, SeeLeo CerenoNo ratings yet
- Organic C CCCC CCCCDocument88 pagesOrganic C CCCC CCCCKugan KishurNo ratings yet
- Zytel® 101L NC010-gbDocument21 pagesZytel® 101L NC010-gbRamiro PredassiNo ratings yet
- January 2012 QP - Unit 4 Edexcel Chemistry A-LevelDocument29 pagesJanuary 2012 QP - Unit 4 Edexcel Chemistry A-LevelMaria KolokasiNo ratings yet
- One Electron Oxidation of 12-Tungstocobalt (III) Ate Microsomal Cytochrome P450Document4 pagesOne Electron Oxidation of 12-Tungstocobalt (III) Ate Microsomal Cytochrome P450Narsinh M. DodiaNo ratings yet
- Exp7 Synthesis of 2-Methyl-2 - Butene ChazaDocument5 pagesExp7 Synthesis of 2-Methyl-2 - Butene ChazaAnthony TannousNo ratings yet
- Reactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Document6 pagesReactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Sandipan SahaNo ratings yet
- All You Need To Know About WhiskeyDocument1 pageAll You Need To Know About Whiskeystelis2No ratings yet
- CHEM1102 Lecture Notes 12Document18 pagesCHEM1102 Lecture Notes 12Callum BiggsNo ratings yet
- Russian Rivers Pliny The ElderDocument2 pagesRussian Rivers Pliny The ElderUbitiqui Quadros QuadrosNo ratings yet
- Alcohol Awareness Answer KeyDocument2 pagesAlcohol Awareness Answer KeyAbdul Jakeem CastanosNo ratings yet