Professional Documents
Culture Documents
Characterization of Coconut Shell Ash For Potentia
Uploaded by
LuisOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Characterization of Coconut Shell Ash For Potentia
Uploaded by
LuisCopyright:
Available Formats
P.B Madakson et al.
/ International Journal of Engineering Science and Technology (IJEST)
Characterization of Coconut Shell Ash for
Potential Utilization in Metal Matrix
Composites for Automotive Applications.
P.B Madakson, D.S.Yawas and A. Apasi.
Department of Mechanical Engineering,
Ahmadu Bello University, Samaru, Zaria, Nigeria
Abstract
Coconut shell ash is agricultural waste. The waste is produced in abundance globally and poses risk to health
as well as environment. Thus their effective, conducive and eco-friendly utilization has always been a
challenge for scientific applications. This paper mainly deals with identification of characteristics of coconut
shell ash using spectroscopic and microscopic analysis. Density, Particle size, Refractoriness, SEM, XRD,
XRF and FTIR spectroscopic methods were used for the characterization of the coconut shell ash. The results
were compared and it was observed that the ash possesses nearly same chemical phases and other functional
groups as reinforcement like fly ash, rice husk ash, bagasse ash that have been in Metal Matrix Composites
(MMCs) specifically for automobile applications. Hence, coconut shell ash can be used as a low cost
reinforcement in Metal Matrix Composites (MMCs).
Key Words: Density; microstructure; particle size; refractoriness
1. Introduction
Researches all over the world today are focusing on ways of utilizing, either industrial or agricultural wastes as a
source of raw materials for the industry. These wastes utilization would not only be economical, but may also
result to foreign exchange earning and environmental pollution control[1-2]
Metal matrix composites (MMCs) posses significantly improved properties including high specific strength
specific modulus, damping capacity and good wear resistance compared to unreinforced alloys[2-4]. Similarly,
there has been an increasing interest in composites containing low density and low cost reinforcements. Among
various discontinuous dispersoids used are fly ash, red mud, Rice husk ash[1-5] are some of the most
inexpensive and low density reinforcement available in large quantities as solid waste by- product.
Coconut shell is an agricultural waste and is available in very large quantities throughout the tropical
countries of the world. Moreover, coconut is becoming an important agricultural product for tropical countries
around the world as a new source of energy-biofuel[6]. Previously, coconut shell was burnt as a means of solid
waste disposal which contributed significantly to CO2 and methane emissions [6]. However as the cost of fuel
oil, natural gas and electricity supply has increased and become erratic, coconut shell has come to be regarded as
source of fuel rather than refuse. Presently, the Nigeria coconut shell is used as a source of fuel for the boilers,
and residual coconut shell is disposed off as gravel for plantation roads maintenance. Black smiths also buy the
coconut shell as fuel material in their casting and forging operations[6].
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1190
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
Bamgboye and Jekayinfa[6] regretted that 90% of coconut (empty fruit bunches, fibers, fronds, trunks,
shell) was discarded as waste and either burned in the open air or left to settle in waste ponds. This way the
coconut processing industries waste according to him contributed significantly to CO2 and methane emissions.
Based on economic as well as environmental related issues, efforts should be directed world wide towards
coconut management issues i.e. of utilization, storage and disposal. Different avenues of coconut shell
utilization are more or less known but none of them have so far proved to be economically viable or
commercially feasible. Hence, the objective of this present work is to characterize coconut shell in order to
explore its use in metal matrix composites.
2. Materials and Method
2.1 Material
The coconut shell used in this work was obtained from a coconut seller in Kaduna, Kaduna state of
Nigeria. The photograph of the coconut shell is shown in Plate 1.
(a) Coconut shell (b) Crushed coconut shell
Plate 1: Photograph of the Coconut shell.
2.2 Equipment
Equipment used in this research are- electrical resistance furnace, X-ray diffractometer (XRD),
Scanning electron microscope with energy dispersive spectrometer (SEM/EDS) Machine, X-ray fluorescent
XRF
2.3 Methods
2.3.1 The processing of the coconut shell (Carbonization)
The coconut shell was grinded to form coconut shell powder, the powder was packed in a graphite
crucible and fired in electric resistance furnace at temperature of 1300oC to form coconut shell ash (CSAp)(see
Plate 2 ).
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1191
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
(a) Coconut shell powder (b) Coconut shell ash
Plate 2: Photograph of coconut shell ash
2.3.2 Particles size analysis
The particle size analysis of the coconut shell ash particles was carried out in accordance with
BS1377:1990[7]. About 100g of the coconut shell ash particles was placed unto a set of sieves arranged in
descending order of fineness and shaken for 15minutes which is the recommended time to achieve complete
classification. The weight retained on each sieve was taken and expressed as percentages of the total sample
weight. From the weight retained, the grain fineness number (AFS) was computed [8].
2.3.3 Density Measurement
Density measurements were carried out on the coconut ash sample using Archimedes’s principle. The
buoyant force on a submerged object is equal to the weight of the fluid displaced. This principle is useful for
determining the volume and therefore the density of an irregularly shaped object by measuring its mass in air and
its effective mass when submerged in water (density = 1 gram/cc). This effective mass under water was its
actual mass minus the mass of the fluid displaced. The difference between the real and effective mass therefore
gives the mass of water displaced and allows the calculation of the volume of the irregularly shaped object. The
mass divided by the volume thus determined gives a measure of the average density of the sample [8].
2.3.4 Refractoriness
The Pyrometric Cone Equivalent (PCE) as recommended by ASTM Test C-24 was used in the
determination of the refractoriness of the sample [2].
2.3.5 Mineralogical Characterization of the Coconut Shell ash
Mini Pal compact energy dispersive X-ray spectrometer (XRF) was used for the elemental analysis of
the coconut shell ash. The system is controlled by a PC running the dedicated Mini Pal analytical software [8].
The XRD analysis of the coconut shell ash was carried out using Philips X-ray diffractometer. The
o
X-ray diffractograms was taken using Cu Kα radiation at scan speed of 3 / min. The samples were rotated at
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1192
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
precisely one-half of the angular speed of the receiving slit, so that a constant angle between the incident and
reflected beams is maintained. The receiving slit is mounted in front of the counter on the counter tube arm, and
behind it is usually fixed a scatter slit to ensure that the counter receives radiation only from the portion of the
specimen illuminated by the primary beam. The intensity diffracted at the various angles was recorded
automatically on a chart and the appropriate (Ө) and (d) values were then obtained[7-9].
2.3.6 Micro structural Analysis
The microstructure and the chemical compositions of the phases present in the coconut shell ash was
TM
studied using a JOEL JSM 5900LV Scanning Electron Microscope equipped with an Oxford INCA
Energy Dispersive Spectroscopy system. The s a m p l e was placed on sample holder and the images were
captured under various magnifications. Prior to it, sample was applied with the gold coating to avoid charge
effect, so to obtain clear images. The SEM was operated at an accelerating voltage of 5 to 20 kV[2].
2.3.7 Fourier Transform Infrared Spectroscopy (FTIR)
FTIR-8400S Fourier transform infrared spectrophotometer (SHIMADZU ) was used for the functional
groups present in the coconut shell ash. Spectrometer and detector, capable of measuring functional group to
the predetermined minimum detectable level. The system include a personal computer with compatible software
that provides real-time updates of the spectral profile during sample collection and spectral collection using
FTIR system using 1 cm-1 resolution, 22 meter path length, and a broad band MCT detector. The Data analysis
was performed using appropriate reference spectra whose concentrations can be verified using CTS spectra[7].
3.0 Result And Discussion
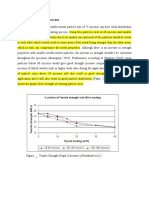
From the particle size analysis results, it is shown that, the coconut shell ash has a Grain Fineness Number
(GFN) of 75.08. The sample can be considered to be fine as GFN value of 100 is ranked the finest. Also, the
sample can be considered to have met the AFS specification since four sieve-size, has the bulk of the retained
sample on four consecutive sieves corresponding to 355μm, 180μm, 125μm, and 63μm size fractions
respectively [2].
The density of the coconut shell ash is 2.05g/cm3 which means that coconut shell ash is very light material.
The value obtained fall within the range of density of fly ash, bagasse and silica which is 1.8 and 2.2 g/cm3
respectively [1, 2] currently used in metal matrix composites
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1193
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
The coconut shell ash was observed to have Seger Cone No. 22, with equivalent temperature of 1500oC.
This means coconut shell ash can withstand operating temperature of 1500oC without load [1-4].
The XRD pattern(see Figure 1) obtained reveal that, the major diffraction peaks are 20.75°, 10.22° and
35.40o and their inter-planar distance, 3.87Å, 8.66 Å and 2.54Å, and their relative intensity of X-ray
scattering are 100.00, 61.90 and 8.44 and phases at these peaks as Quartz(SiO2), Cordierite, syn (Mg2
Al4Si5O18) and Moissanite(SiC), while each of these phases have a score of 44, 32 and 16
respectively(see Table 1).
C ounts
Mg2 Al4 Si5 O18
Si O2; Mg2 Al4 Si5 O18; Si O2
Mg2 Al4 Si5 O18
Si O2; Mg2 Al4 Si5 O18; Si O2
d2_ 00940 -ver3 .raw
Mg2 Al4 Si5 O18
10 00
Si O2; Si O2
Si O2; Si C
Si C
Si O2; Si C
5 00
0
20 30 40 50 60 70 80 90
P ositio n [°2 Theta]
Figure 1: XRD pattern of Coconut shell ash
Table 1: Identified patterns list of coconut shell ash
Visible Ref. Code Score Compound Name Displacement Scale Factor Chemical Formula
[°2Th.]
* 00-043-0596 44 Silicon Oxide 0.000 0.912 Si O2
* 00-013-0294 32 Cordierite, syn 0.000 0.590 Mg2 Al4 Si5 O18
* 01-089-1961 26 Quartz low, 0.000 0.642 Si O2
dauphinee-twinned
* 01-075-1541 16 Moissanite 0.000 0.207 Si C
6\ITH\RG
The results showed that SiO2 has the highest percentage of all the compound and element present as
revealed by the XRD analysis. Complete Mineralogical analysis carried out by X-ray diffraction also
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1194
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
revealed that the ash contains each of these elements C, O, Mg, Al, Si" Fe, Na, K, Zn and none of these
elements H, He, Li, Be, B, N, F, Ne, P, S, Cl, Ga, Ge, As, Se, Br, Kr, Rb, Sr, Y, Nb, Mo, Tc, Ru, Rh, Pd, Ag,
Cd, In, Sn, Sb, Te, I, Xe, Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, which
means that with the absence of all these other elements the coconut shell ash may not contain radioactive
materials. This is in par with the earlier of other biomass by[1-2]. The XRD result showed that both SiO2
and SiC have a fine structure, the former having a finer one. This could b e associated with pore size
[2].
The XRF chemical composition of the coconut shell ash is represented in Table 2. X RF ana lysis
confirmed that SiO2, Al2O3, MgO and Fe2O3 were found to be major constituents of the ash. Silicon
dioxide, iron oxide and alumina are known to be among the hardest substances. Some other oxides viz. CaO,
K2O, Na2O and MnO were also found to be present in traces. The presence of hard elements like SiO2,
Al2O3 a n d Fe2O3 suggested that, the coconut shell ash can be use as particulate reinforcement in various
metal matrixes. This result of XRF is in agreement with the result of XRD obtained. Therefore, the present
work suggests the possibility of using coconut shell ash as particulate in metal matrix composites since the
chemical composition has close similarity with the XRF analysis of rick husk ash, bagasse ash and fly ash
currently used in metal matrix composites[1-4].
Table 2: XRF analysis of Coconut shell ash
Element Al2O3 CaO Fe2O3 K2O MgO Na2O SiO2 MnO ZnO
% 15.6 0.57 12.4 0.52 16.2 0.45 45.05 0.22 0.3
Particle Morphology of coconut shell ash can be seen in back scattered electron (BSE) as shown in
Figure 2. Coconut shell ash particles were observed to be solid in nature, but irregular in size. Some
spherical shape particles can also be seen in the Figure 2. The chemical analysis of the coconut shell
ash morphology consists mainly of Si, C, O, Mg, Al with small amounts of Fe as shown in the EDS
scan (see Figure 2). The results are consistent with XRD and XRF analysis of other biomass by[1-5].
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1195
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
Figure 2: SEM/EDS spectrum of Coconut shell ash
Mainly twenty peaks were detected in the FTIR Analysis of the coconut shell ash as visible in Figure 3.
These peaks are shown in Table 3. This result has shown that the presence of quartz in the original ash gives
rise in the IR spectrum to a series of bands located at 1132 and 443.64 cm-1. The presence of mullite, in
turn, is responsible for a series of bands at around 3797 cm-1. The presence of carbon group is present in
series of bands at around 4091.15-4617.74 cm-1. Quartz, mullite and the vitreous phase of the ash overlap in
the area between 1220 cm-1 and 1434.12cm-1. Hence Quartz, Mullite, carbon and vitreous phases are
confirmed to be present. More over the peaks in treated and untreated cases does not show any variations.
This is in agreement with the earlier work of [4, 9].
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1196
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
Figure 3: FTIR spectrum of Coconut shell ash
Table 3: Identification Peaks of the FTIR analysis
4. Conclusions
From the analysis of the results and discussion given above, the following conclusions can be made.
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1197
P.B Madakson et al. / International Journal of Engineering Science and Technology (IJEST)
1) XRD analysis of the coconut shell ash reveals Silicon Oxide: (SiO2), Corderite,syn:(Mg2
AlSi5O18),Quartz:(SiO2) and Moissanite (SiC) as the primary compound with SiO2 as the highest
percentage of all the compound and element present.
2) XRF studies revealed the presence of hard element like SiO2, Al2O3, MgO and Fe2O3 as major
constituents which can be used as particulate reinforcements in MMCs for automobile applications.
3) FTIR graphs showed that Quartz, Mullite and Vitereous, carbon phases were present in coconut shell
ash powder and proposed to use coconut shell ash as particulate reinforcement in MMCs.
4) The coconut shell ash can withstand a temperature of up to 1500oC with a density of 2.05g/cm3. That
means this ash can be use in production light weight MMCs component with good thermal resistance.
5.0 REFERENCES
[1] Bienia J, Walczak .M, Surowska, B, Sobczaka J (2003): Microstructure and corrosion behaviour of aluminum fly ash composites,
Journal of Optoelectronics and Advanced Materials, 5, No. 2,493 – 502.
[2] Aigbodion .V. S., Hassan S. B., Ause .T. and Nyior,.G.B(2010):. Potential Utilization of Solid Waste (Bagasse Ash), Journal of
Minerals & Materials Characterization & Engineering, Vol. 9, No.1, pp.67-77,
[3] Siva Prasad. D and Rama Krishna. A(2011): Production and Mechanical Properties of A356.2 /RHA Composites, International Journal
of Advanced Science and Technology Vol. 33, 51-58
[4] Arun Kumar M. B and Swamy R. P(2011): Evaluation of Mechanical Properties of Al6061, Flyash And E-Glass Fiber Reinforced
Hybrid Metal Matrix Composites, ARPN Journal of Engineering and Applied Sciences, VOL. 6, NO. 5,40-44.
[5] Naresh P(2006): Development and characterization of metal matrix composite using red Mud an industrial waste for wear
resistant applications, PHD thesis Department of Mechanical Engineering, National Institute of Technology Rourkela -769 008
(India), January, pp23-34.
[6] Bamgboye A. Isaac and Jekayinfa .S. O(2006): Energy Consumption Pattern in Coconut Processing Operations. Agricultural
Engineering International: the CIGR Journal Manuscript EE 05 013. Vol. VIII.
[7] Rajan T.P.D, Pillai R.M, Pai B.C., Satyanarayana K.G, Rohatgi, P.K (2007): Fabrication and characterization of Al–7Si–
0.35Mg/fly ash metal matrix composites processed by different stir casting routes, Composites Science and Technology, 67,
3369–3377.
[8] Aigbodion. V.S(2010): Potential of using bagasses ash particle in Metal Matrix Composite, PHD Department of Metallurgical and
Materials Engineering, Ahmadu Bello University, Samaru, Zaria, Nigeria
[9] Ejilofor J. U and Reddy R.G(1997): Developments in the processing and properties of particulate Al-Si Composites, Jom publication
of minerals metals and materials society 49 (11), pp 31-37.
ISSN : 0975-5462 Vol. 4 No.03 March 2012 1198
You might also like
- VOCABULARY 5 TOWNS AND BUILDINGS Part BDocument4 pagesVOCABULARY 5 TOWNS AND BUILDINGS Part BELENANo ratings yet
- Freebie The Great Composerslapbookseries ChopinDocument24 pagesFreebie The Great Composerslapbookseries ChopinAnonymous EzNMLt0K4C100% (1)
- Gallup-12-Questions With AnswersDocument6 pagesGallup-12-Questions With Answersjeanette4hijadaNo ratings yet
- Specific Gravity of Palm Kernel ShellDocument5 pagesSpecific Gravity of Palm Kernel ShellChidi HenryNo ratings yet
- Optimizing Purity and Recovery of Hydrogen From Syngas by Equalized Pressure Swing Adsorption Using Palm Kernel Shell Activated Carbon AdsorbentDocument19 pagesOptimizing Purity and Recovery of Hydrogen From Syngas by Equalized Pressure Swing Adsorption Using Palm Kernel Shell Activated Carbon AdsorbentMohamad HafizeeNo ratings yet
- Shells 3Document8 pagesShells 3Donna HayesNo ratings yet
- Biological Coating of Paper Using Silver NanoparticlesDocument5 pagesBiological Coating of Paper Using Silver NanoparticlesElizabeth PeraltaNo ratings yet
- Foods 12 00746Document14 pagesFoods 12 00746getahun esubalewNo ratings yet
- Characterization of Cellulose NanocrystaDocument8 pagesCharacterization of Cellulose Nanocrystauntukdownload downloadNo ratings yet
- Materials Chemistry and PhysicsDocument11 pagesMaterials Chemistry and Physicsimran shaukatNo ratings yet
- Green Technology Extraction and Characterisation of Silica Nanoparticles From Palm Kernel Shell Ash Via Sol-GelDocument7 pagesGreen Technology Extraction and Characterisation of Silica Nanoparticles From Palm Kernel Shell Ash Via Sol-GelMhd Ihsan NabilNo ratings yet
- DPC 2016 8 15 227 232Document7 pagesDPC 2016 8 15 227 232Mutia RezaNo ratings yet
- Isolation and Characterization of Nanocrystalline Cellulose From Roselle-Derived Microcrystalline CelluloseDocument10 pagesIsolation and Characterization of Nanocrystalline Cellulose From Roselle-Derived Microcrystalline CelluloseHidayah AriffinNo ratings yet
- kian2018Document41 pageskian2018nafsiyah xyzNo ratings yet
- 15 Makale-Int J Sec MetaboliteVol 4-3-pp 77-84-2017 PDFDocument8 pages15 Makale-Int J Sec MetaboliteVol 4-3-pp 77-84-2017 PDFMaharani PuspaningrumNo ratings yet
- Green Synthesis of Silver Nanoparticles Using An 2017 Saudi Journal of BioloDocument6 pagesGreen Synthesis of Silver Nanoparticles Using An 2017 Saudi Journal of Bioloshiba meike indiraNo ratings yet
- Morphological and Micro-Grain Structural Analyses of Pretreated Substrates (Sawdust and Poultry Dung) For Potential Use in Enhanced Biogas ProductionDocument9 pagesMorphological and Micro-Grain Structural Analyses of Pretreated Substrates (Sawdust and Poultry Dung) For Potential Use in Enhanced Biogas ProductionDANIEL IDUSUYINo ratings yet
- Biosynthesis, Characterization and Photocatalytic Activities of Ag-Cu Bimetallic Nanoparticles Derived From Mukia Maderaspatana Leaf ExtractDocument7 pagesBiosynthesis, Characterization and Photocatalytic Activities of Ag-Cu Bimetallic Nanoparticles Derived From Mukia Maderaspatana Leaf ExtractIJRASETPublicationsNo ratings yet
- Materials Today: Proceedings: K.A. Ganure, B.L. Shinde, U.M. Mandle, L.A. Dhale, R.M. Tigote, K.S. LoharDocument8 pagesMaterials Today: Proceedings: K.A. Ganure, B.L. Shinde, U.M. Mandle, L.A. Dhale, R.M. Tigote, K.S. LoharJuancho PachonNo ratings yet
- Jurnal 2 KoagulanDocument8 pagesJurnal 2 KoagulanfauzanNo ratings yet
- Composites: Part B: H.P.S. Abdul Khalil, H.M. Fizree, A.H. Bhat, M. Jawaid, C.K. AbdullahDocument10 pagesComposites: Part B: H.P.S. Abdul Khalil, H.M. Fizree, A.H. Bhat, M. Jawaid, C.K. Abdullaheid elsayedNo ratings yet
- Research ArticleDocument11 pagesResearch ArticleXyNo ratings yet
- IJTech CE 1741 Synthesis of Nanosilica From Boiler Ash in The SugDocument14 pagesIJTech CE 1741 Synthesis of Nanosilica From Boiler Ash in The SugNAZWA SALSABILLAHNo ratings yet
- Puspitasari 2019 IOP Conf. Ser. Mater. Sci. Eng. 515 012104Document8 pagesPuspitasari 2019 IOP Conf. Ser. Mater. Sci. Eng. 515 012104Cassia MontiNo ratings yet
- Biomass Residue Characterization For Their Potential Application As BiofuelsDocument9 pagesBiomass Residue Characterization For Their Potential Application As Biofuelsmir shifayatNo ratings yet
- Kwame (2022)Document9 pagesKwame (2022)Antony NeciosupNo ratings yet
- Oil PalmDocument16 pagesOil Palmedison avvaiNo ratings yet
- Production of Particle Boards From Corn Cobs and Cassava Stalks: Optimisation of Mechanical Properties Using Response Surface MethodologyDocument10 pagesProduction of Particle Boards From Corn Cobs and Cassava Stalks: Optimisation of Mechanical Properties Using Response Surface MethodologyVictoria Roselle AlmarezNo ratings yet
- Characterization of Green Synthesized Silver Nanoparticle of Commiphora Caudata BarkDocument2 pagesCharacterization of Green Synthesized Silver Nanoparticle of Commiphora Caudata BarkInternational Jpurnal Of Technical Research And ApplicationsNo ratings yet
- Renewable Energy: Alireza Bazargan, Majid Bazargan, Gordon MckayDocument9 pagesRenewable Energy: Alireza Bazargan, Majid Bazargan, Gordon MckayReem The AuthorNo ratings yet
- Nanomaterials 08 01069Document21 pagesNanomaterials 08 01069ANSHIDA ROUNA A N IMS21031No ratings yet
- Properties of Calcium Carbonate Nanoparticles from Snail ShellsDocument10 pagesProperties of Calcium Carbonate Nanoparticles from Snail ShellsFaissal El KhazantiNo ratings yet
- Physical Properties of Coconut Shell NanoparticlesDocument17 pagesPhysical Properties of Coconut Shell NanoparticlesChoon Zhe ShyiNo ratings yet
- Case Studies in Thermal Engineering: SciencedirectDocument6 pagesCase Studies in Thermal Engineering: SciencedirectPerdinan SinuhajiNo ratings yet
- Ijcps Ijcps Ijcps Ijcps: International Journal of Chemical and Pharmaceutical Sciences 2014, Sep., Vol. 5Document7 pagesIjcps Ijcps Ijcps Ijcps: International Journal of Chemical and Pharmaceutical Sciences 2014, Sep., Vol. 5briyan rhNo ratings yet
- BactericidalEvaluationofNano coatedCottonFabrics HananlastPaperDocument9 pagesBactericidalEvaluationofNano coatedCottonFabrics HananlastPaperSawitchaya SinprommaNo ratings yet
- Ijmet: ©iaemeDocument8 pagesIjmet: ©iaemeIAEME PublicationNo ratings yet
- 387-OushabiDocument8 pages387-Oushabiziadslimane1995No ratings yet
- Agricultural Waste Biomass Hydrochars CharacterizedDocument7 pagesAgricultural Waste Biomass Hydrochars CharacterizedHasibul HasanNo ratings yet
- Dynamic Mechanical and Thermal Properties of Jute Nano Fibre Reinforced Polymer CompositeDocument6 pagesDynamic Mechanical and Thermal Properties of Jute Nano Fibre Reinforced Polymer CompositeMukul AzadNo ratings yet
- MY Letter Guideliness and Application Form For BE & ME Proje 2017-20Document7 pagesMY Letter Guideliness and Application Form For BE & ME Proje 2017-20PRAKASH KUMARNo ratings yet
- penerbitRPMMEVol 1no 1-44-55Document12 pagespenerbitRPMMEVol 1no 1-44-55Shaina Mariz PanaliganNo ratings yet
- Green Synthesis of Iron Oxide Nanoparticles Using Simarouba Glauca Leaf Extract and Application in Textile Effluent TreatmentDocument9 pagesGreen Synthesis of Iron Oxide Nanoparticles Using Simarouba Glauca Leaf Extract and Application in Textile Effluent TreatmentIJRASETPublicationsNo ratings yet
- Kouassi Brou Guillaume, Preparation of Activated Carbon From Cashew Nut Shells For Water PurificationDocument8 pagesKouassi Brou Guillaume, Preparation of Activated Carbon From Cashew Nut Shells For Water PurificationBrou Guillaume KOUASSINo ratings yet
- Aniagor2020 - Relational Description of An Adsorption System Based On IsothermDocument26 pagesAniagor2020 - Relational Description of An Adsorption System Based On Isothermco.aniagorNo ratings yet
- 2012 Separation and Characterization of New CellulosicDocument17 pages2012 Separation and Characterization of New CellulosicMario DzulNo ratings yet
- Eco-Friendly Synthesis of Cuprous Oxide (Cu2O) Nanoparticles and Improvement of Their Solar PhotocatalyticDocument5 pagesEco-Friendly Synthesis of Cuprous Oxide (Cu2O) Nanoparticles and Improvement of Their Solar PhotocatalyticNguyễn Đắc DiệnNo ratings yet
- Comparative Calorific Analyzes of Coconut Shell and Durian Fruit Peel by Using Differential Scanning CalorimetryDocument9 pagesComparative Calorific Analyzes of Coconut Shell and Durian Fruit Peel by Using Differential Scanning CalorimetryAjuwiNo ratings yet
- 1 s2.0 S2238785420316653 MainDocument14 pages1 s2.0 S2238785420316653 MainVishnu PriyaNo ratings yet
- Budhi_2018_IOP_Conf._Ser.__Earth_Environ._Sci._105_012063Document7 pagesBudhi_2018_IOP_Conf._Ser.__Earth_Environ._Sci._105_012063nafsiyah xyzNo ratings yet
- Sanni2018 Article AnApproachToOptimizePre-AnnealDocument10 pagesSanni2018 Article AnApproachToOptimizePre-AnnealAmal SNo ratings yet
- Reuse of Eggshell Waste and Recycled Glass in TheDocument12 pagesReuse of Eggshell Waste and Recycled Glass in TheJohn Michael EcaranNo ratings yet
- 4-A Review of Solar Thermal Desalination Using NanotechnologyDocument9 pages4-A Review of Solar Thermal Desalination Using NanotechnologymoumineensaNo ratings yet
- Comparative Study of Thermal Insulation Boards From Leaf and Bark Fibres of Camel'S Foot (L.)Document7 pagesComparative Study of Thermal Insulation Boards From Leaf and Bark Fibres of Camel'S Foot (L.)SheenaNo ratings yet
- The Kinetics of The Adsorption Process of CR (VI) in Aqueous Solution Using Neem Seed Husk (Azadirachta Indica) Activated CarbonDocument13 pagesThe Kinetics of The Adsorption Process of CR (VI) in Aqueous Solution Using Neem Seed Husk (Azadirachta Indica) Activated CarbonNórida Pájaro GómezNo ratings yet
- Viena 2019 J. Phys. Conf. Ser. 1232 012005Document9 pagesViena 2019 J. Phys. Conf. Ser. 1232 012005latifhamdi619No ratings yet
- Kasmuri 2022 IOP Conf. Ser. Earth Environ. Sci. 1019 012048Document14 pagesKasmuri 2022 IOP Conf. Ser. Earth Environ. Sci. 1019 012048Joven Rafael MesaNo ratings yet
- Paracetamol GroundnutShellsDocument11 pagesParacetamol GroundnutShellsRajesh KannanNo ratings yet
- Synthesis and Characterization of Some Modified Natural Nanocomposite Polymers and Study Their Thermal and Mechanical PropertiesDocument12 pagesSynthesis and Characterization of Some Modified Natural Nanocomposite Polymers and Study Their Thermal and Mechanical PropertiesIJAR JOURNALNo ratings yet
- Preparation and Characterization of Modified Cornc2Document28 pagesPreparation and Characterization of Modified Cornc2Mad Ramirez SantosNo ratings yet
- Optimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using ZNCLDocument7 pagesOptimization of Conditions For The Preparation of Activated Carbon From Mango Nuts Using ZNCLIJERDNo ratings yet
- Nanocrystalline Materials: Their Synthesis-Structure-Property Relationships and ApplicationsFrom EverandNanocrystalline Materials: Their Synthesis-Structure-Property Relationships and ApplicationsNo ratings yet
- Heterogeneous Nanocomposite-Photocatalysis for Water PurificationFrom EverandHeterogeneous Nanocomposite-Photocatalysis for Water PurificationNo ratings yet
- Comparative Assessment of Mechanical ProDocument11 pagesComparative Assessment of Mechanical ProLuisNo ratings yet
- Revision For Effect of Particle SizeDocument4 pagesRevision For Effect of Particle SizeLuisNo ratings yet
- Al-1100-CSA Composite WearDocument42 pagesAl-1100-CSA Composite WearLuisNo ratings yet
- Development and Analysis of Fly Ash Reinforced Aluminum Alloy Matrix CompositesDocument6 pagesDevelopment and Analysis of Fly Ash Reinforced Aluminum Alloy Matrix CompositesLuisNo ratings yet
- Compilation Mark 2.5Document102 pagesCompilation Mark 2.5LuisNo ratings yet
- (Iihfw2I) O/$Vk+/Eulg5Hlqirufhphqw2Q0Hfkdqlfdo3Urshuw/ $Qg'Hqvlw/2I$Oxplqlxp$OorDocument9 pages(Iihfw2I) O/$Vk+/Eulg5Hlqirufhphqw2Q0Hfkdqlfdo3Urshuw/ $Qg'Hqvlw/2I$Oxplqlxp$OorSwanand kulkarniNo ratings yet
- Coco Shell BacteriaDocument2 pagesCoco Shell BacteriaLuisNo ratings yet
- Fabrication and Mechanical Characterization of Aluminium (6061) With Conventionally Prepared BamboocharcoalDocument11 pagesFabrication and Mechanical Characterization of Aluminium (6061) With Conventionally Prepared BamboocharcoalLuisNo ratings yet
- Mechanical Properties of Fly Ash Reinforced Aluminium 6061 CompositeDocument5 pagesMechanical Properties of Fly Ash Reinforced Aluminium 6061 CompositeLuisNo ratings yet
- Al6061 With Bamboo CharDocument11 pagesAl6061 With Bamboo CharLuisNo ratings yet
- Fcra Act 2010 PDFDocument2 pagesFcra Act 2010 PDFKateNo ratings yet
- Selkie - Set and PropsDocument31 pagesSelkie - Set and Propsapi-620650833No ratings yet
- HR 111 - Pal RetrenchmentDocument2 pagesHR 111 - Pal RetrenchmentanakpawispartylistNo ratings yet
- ?PMA 138,39,40,41LC Past Initials-1Document53 pages?PMA 138,39,40,41LC Past Initials-1Saqlain Ali Shah100% (1)
- 2018 Gauss 7 ContestDocument4 pages2018 Gauss 7 ContestpriyagvNo ratings yet
- Chemicals Companies UK 2009Document21 pagesChemicals Companies UK 2009Dharmendra B MistryNo ratings yet
- Gerunds and Infinitives 8897Document3 pagesGerunds and Infinitives 8897aura lucy estupiñan gutierrezNo ratings yet
- A Street Car Named Desire (Penguin)Document18 pagesA Street Car Named Desire (Penguin)the_perfectionistNo ratings yet
- City of Carmel-By-The-Sea Website RFP 2017Document27 pagesCity of Carmel-By-The-Sea Website RFP 2017L. A. PatersonNo ratings yet
- 7 Introduction To Life SkillsDocument2 pages7 Introduction To Life SkillsMailat SoranaNo ratings yet
- Financial AnalysisDocument4 pagesFinancial AnalysisRoel P. Dolaypan Jr.No ratings yet
- What is Economics? - The study of production, distribution and consumption /TITLEDocument37 pagesWhat is Economics? - The study of production, distribution and consumption /TITLEparthNo ratings yet
- The Small Trees High Productivity Initiative: Principles and Practice in High Density Orchard DesignDocument1 pageThe Small Trees High Productivity Initiative: Principles and Practice in High Density Orchard DesignPutchong SaraNo ratings yet
- Vasitars PVT Limited - Pipeline RepairsDocument12 pagesVasitars PVT Limited - Pipeline RepairsPavan_yoyo100% (1)
- Taketo SUZUKIDocument3 pagesTaketo SUZUKIJane DeckerNo ratings yet
- PV Launching Digital Wallet AccountDocument1 pagePV Launching Digital Wallet Accountbet47No ratings yet
- 1.1 Network MarketingDocument92 pages1.1 Network Marketingpriti100No ratings yet
- HCPN Certification Agreement, Partner Agreement-HUAWEI CLOUDDocument9 pagesHCPN Certification Agreement, Partner Agreement-HUAWEI CLOUDSolNo ratings yet
- Business Profile (JAO-REN)Document15 pagesBusiness Profile (JAO-REN)Michael T. SebullenNo ratings yet
- Mitra 2018Document4 pagesMitra 2018rahulNo ratings yet
- Chapter 01Document33 pagesChapter 01Michael BANo ratings yet
- Descarga de ScribdDocument4 pagesDescarga de ScribdYoel MartínezNo ratings yet
- Srimad Bhagavatam 2nd CantoDocument25 pagesSrimad Bhagavatam 2nd CantoDeepak K OONo ratings yet
- Reigeluth 1983Document14 pagesReigeluth 1983agssuga100% (1)
- Post Graduate Medical (Government Quota) Course Session:2021 - 2022 List of Candidates Allotted On - 04.03.2022 (Round 2)Document104 pagesPost Graduate Medical (Government Quota) Course Session:2021 - 2022 List of Candidates Allotted On - 04.03.2022 (Round 2)Aravind RaviNo ratings yet
- Scaffold Inspection Checklist FINALDocument2 pagesScaffold Inspection Checklist FINALRhannie GarciaNo ratings yet
- Encoder DecoderDocument6 pagesEncoder Decodermarkcelis1No ratings yet