Professional Documents
Culture Documents
Unit 3 Respiratory Physiology
Uploaded by
Mandeep Debnath0 ratings0% found this document useful (0 votes)
18 views4 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views4 pagesUnit 3 Respiratory Physiology
Uploaded by
Mandeep DebnathCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 4
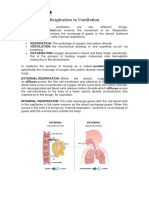
Respiratory Physiology respiration, oxygen and carbon dioxide are exchanged
between the cells and blood vessels.
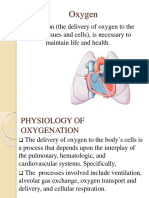
Respiratory physiology is characterized by two linked
processes, oxygenation, and ventilation. Oxygenation Respiration begins at the nose or mouth, where
refers to the addition of oxygen (O2) to the bloodstream oxygenated air is brought in before moving down the
from the air, which is typically at a concentration of 21%, pharynx, larynx, and trachea. The trachea branches into
also known as the fraction of inspired oxygen (FiO2). two bronchi, each leading into a lung. Each bronchus
Ventilation is the clearance of carbon dioxide (CO2) from divides into smaller bronchi, and again into even smaller
the bloodstream after the latter has been generated by tubes called bronchioles. At the end of the bronchioles are
cellular respiration. It can help to think of these two air sacs called alveoli, and this is where gas exchange
processes as entirely separate, although in reality, this occurs.
abstraction breaks down at extremely low minute
ventilation. Common mistakes and misconceptions
● Physiological respiration and cellular respiration
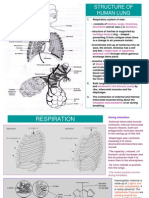
Both processes rely upon air coming down the oral cavity, are not the same. People sometimes use the
into the trachea, through the bronchi, and into the lung word "respiration" to refer to the process of
parenchyma, where blood brought from the pulmonary cellular respiration, which is a cellular process in
artery goes through the pulmonary capillaries. This which carbohydrates are converted into energy.
alveolar-capillary interface is where gas exchange occurs. The two are related processes, but they are not
If too much blood flow relative to oxygenation capacity is the same.
present, it is referred to as shunt physiology. Air exchange ● We do not breathe in only oxygen or breathe out
in areas that do not have sufficient blood supply is known only carbon dioxide. Often the terms "oxygen" and
as dead space physiology. Some amount of both of these "air" are used interchangeably. It is true that the air
is normal: less than 10% of total CO does not participate we breathe in has more oxygen than the air we
in gas exchange and 20% to 30% of total ventilation does breathe out, and the air we breathe out has more
not equilibrate with blood. Increases in shunt fraction carbon dioxide than the air that we breathe in.
occur secondary to asthma, to distention of alveoli from However, oxygen is just one of the gases found in
pulmonary edema or pneumonia, to atelectasis, or to PE, the air we breathe. (In fact, the air has more
where excessive CO flows through nonmetabolized nitrogen than oxygen!)
regions. Dead space ventilation takes place when the ● The respiratory system does not work alone in
alveolar-capillary interface is destroyed by emphysema, transporting oxygen through the body. The
when the CO is low, or when the air over distends the respiratory system works directly with the
alveoli during positive pressure ventilation. circulatory system to provide oxygen to the body.
Oxygen taken in from the respiratory system
moves into blood vessels that then circulate
The respiratory system oxygen-rich blood to tissues and cells.
Transport of Oxygen and Carbon Dioxide in the
Blood
Once the oxygen diffuses across the alveoli, it enters the
bloodstream and is transported to the tissues where it is
unloaded, and carbon dioxide diffuses out of the blood
and into the alveoli to be expelled from the body. Although
gas exchange is a continuous process, the oxygen and
carbon dioxide are transported by different mechanisms.
Transport of Oxygen in the Blood
Although oxygen dissolves in blood, only a small amount
of oxygen is transported this way. Only 1.5 percent of
oxygen in the blood is dissolved directly into the blood
itself. Most oxygen—98.5 percent—is bound to a protein
called hemoglobin and carried to the tissues.
Diagram labeling the major structures of the respiratory
system Hemoglobin
Hemoglobin, or Hb, is a protein molecule found in red
An important structure of respiration is the diaphragm. blood cells (erythrocytes) made of four subunits: two
When the diaphragm contracts, it flattens and the lungs alpha subunits and two beta subunits (Figure 20.19). Each
expand, drawing air into the lungs. When it relaxes, air flows subunit surrounds a central heme group that contains iron
out, allowing the lungs to deflate. and binds one oxygen molecule, allowing each
hemoglobin molecule to bind four oxygen molecules.
The process of physiological respiration includes two Molecules with more oxygen bound to the heme groups
major parts: external respiration and internal respiration. are brighter red. As a result, oxygenated arterial blood
External respiration, also known as breathing, involves where the Hb is carrying four oxygen molecules is bright
both bringing air into the lungs (inhalation) and releasing red, while venous blood that is deoxygenated is darker
air into the atmosphere (exhalation). During internal red.
reach the same hemoglobin saturation level as when the
pH was higher. A similar shift in the curve also results
from an increase in body temperature. Increased
temperature, such as from increased activity of skeletal
muscle, causes the affinity of hemoglobin for oxygen to
be reduced.
Diseases like sickle cell anemia and thalassemia
decrease the blood’s ability to deliver oxygen to tissues
Figure 20.19. The protein inside (a) red blood cells that and its oxygen-carrying capacity. In sickle cell anemia, the
carries oxygen to cells and carbon dioxide to the lungs is shape of the red blood cell is crescent-shaped, elongated,
(b) hemoglobin. Hemoglobin is made up of four and stiffened, reducing its ability to deliver oxygen (Figure
symmetrical subunits and four heme groups. Iron 20.21). In this form, red blood cells cannot pass through
associated with the heme binds oxygen. It is the iron in the capillaries. This is painful when it occurs.
hemoglobin that gives blood its red color. Thalassemia is a rare genetic disease caused by a defect
in either the alpha or the beta subunit of Hb. Patients with
thalassemia produce a high number of red blood cells, but
these cells have lower-than-normal levels of hemoglobin.
It is easier to bind a second and third oxygen molecule to Therefore, the oxygen-carrying capacity is diminished.
Hb than the first molecule. This is because the
hemoglobin molecule changes its shape, or conformation,
as oxygen binds. The fourth oxygen is then more difficult
to bind. The binding of oxygen to hemoglobin can be
plotted as a function of the partial pressure of oxygen in
the blood (x-axis) versus the relative Hb-oxygen saturation
(y-axis). The resulting graph—an oxygen dissociation
curve—is sigmoidal, or S-shaped (Figure 20.20). As the
partial pressure of oxygen increases, the hemoglobin
becomes increasingly saturated with oxygen.
Figure 20.21.
Individuals with sickle cell anemia have crescent-shaped
red blood cells. (credit: modification of work by Ed Uthman;
scale-bar data from Matt Russell)
Transport of Carbon Dioxide in the Blood
Figure 20.20. The oxygen dissociation curve demonstrates Carbon dioxide molecules are transported in the blood
that, as the partial pressure of oxygen increases, more from body tissues to the lungs by one of three methods:
oxygen binds hemoglobin. However, the affinity of dissolution directly into the blood, binding to hemoglobin
hemoglobin for oxygen may shift to the left or the right or carried as a bicarbonate ion. Several properties of
depending on environmental conditions. carbon dioxide in the blood affect its transport. First,
carbon dioxide is more soluble in blood than oxygen.
The kidneys are responsible for removing excess H+ ions About 5 to 7 percent of all carbon dioxide is dissolved in
from the blood. If the kidneys fail, what would happen to the plasma. Second, carbon dioxide can bind to plasma
blood pH and to hemoglobin affinity for oxygen? proteins or can enter red blood cells and bind to
hemoglobin. This form transports about 10 percent of the
Factors That Affect Oxygen Binding carbon dioxide. When carbon dioxide binds to
The oxygen-carrying capacity of hemoglobin determines hemoglobin, a molecule called carbaminohemoglobin is
how much oxygen is carried in the blood. In addition to formed. The binding of carbon dioxide to hemoglobin is
PO2, other environmental factors and diseases can affect reversible. Therefore, when it reaches the lungs, the
oxygen-carrying capacity and delivery. carbon dioxide can freely dissociate from the hemoglobin
and be expelled from the body.
Carbon dioxide levels, blood pH, and body temperature
affect oxygen-carrying capacity (Figure 20.20). When Third, the majority of carbon dioxide molecules (85
carbon dioxide is in the blood, it reacts with water to form percent) are carried as part of the bicarbonate buffer
bicarbonate (HCO−3) and hydrogen ions (H+). As the level system. In this system, carbon dioxide diffuses into the
of carbon dioxide in the blood increases, more H+ is red blood cells. Carbonic anhydrase (CA) within the red
produced and the pH decreases. This increase in carbon blood cells quickly converts carbon dioxide into carbonic
dioxide and subsequent decrease in pH reduces the acid (H2CO3). Carbonic acid is an unstable intermediate
affinity of hemoglobin for oxygen. The oxygen dissociates molecule that immediately dissociates into (HCO−3) and
from the Hb molecule, shifting the oxygen dissociation hydrogen (H+) ions. Since carbon dioxide is quickly
curve to the right. Therefore, more oxygen is needed to converted into bicarbonate ions, this reaction allows for
the continued uptake of carbon dioxide into the blood Summary
down its concentration gradient. It also results in the Hemoglobin is a protein found in red blood cells that are
production of H+ ions. If too much H+ is produced, it can comprised of two alpha and two beta subunits that
alter blood pH. However, hemoglobin binds to the free H+ surround an iron-containing heme group. Oxygen readily
ions and thus limits shifts in pH. The newly synthesized binds this heme group. The ability of oxygen to bind
bicarbonate ion is transported out of the red blood cell increases as more oxygen molecules are bound to heme.
into the liquid component of the blood in exchange for a Disease states and altered conditions in the body can
chloride ion (Cl–). affect the binding ability of oxygen, and increase or
decrease its ability to dissociate from hemoglobin.
When the blood reaches the lungs, the bicarbonate ion is Carbon dioxide can be transported through the blood via
transported back into the red blood cell in exchange for three methods. It is dissolved directly in the blood, bound
the chloride ion. The H+ ion dissociates from the to plasma proteins or hemoglobin, or converted into
hemoglobin and binds to the bicarbonate ion. This bicarbonate. The majority of carbon dioxide is transported
produces the carbonic acid intermediate, which is as part of the bicarbonate system. Carbon dioxide
converted back into carbon dioxide through the enzymatic diffuses into red blood cells. Inside, carbonic anhydrase
action of CA. The carbon dioxide produced is expelled converts carbon dioxide into carbonic acid (H2CO3),
through the lungs during exhalation. which is subsequently hydrolyzed into bicarbonate
(HCO−3) and H+. The H+ ion binds to hemoglobin in red
blood cells, and bicarbonate is transported out of the red
blood cells in exchange for a chloride ion. This is called
the chloride shift. Bicarbonate leaves the red blood cells
The benefit of the bicarbonate buffer system is that and enters the blood plasma. In the lungs, bicarbonate is
carbon dioxide is “soaked up” into the blood with little transported back into the red blood cells in exchange for
change to the pH of the system. This is important chloride. The H+ dissociates from hemoglobin and
because it takes only a small change in the overall pH of combines with bicarbonate to form carbonic acid with the
the body for severe injury or death to result. The presence help of carbonic anhydrase, which further catalyzes the
of this bicarbonate buffer system also allows for people reaction to convert carbonic acid back into carbon dioxide
to travel and live at high altitudes: When the partial and water. The carbon dioxide is then expelled from the
pressure of oxygen and carbon dioxide change at high lungs.
altitudes, the bicarbonate buffer system adjusts to
regulate carbon dioxide while maintaining the correct pH Explain the transport of oxygen and carbon dioxide in the
in the body.
blood?
Carbon Monoxide Poisoning Ans- The transport of oxygen in blood takes place
While carbon dioxide can readily associate and dissociate through RBCs. during inhalation, oxygen is taken into the
from hemoglobin, other molecules such as carbon lungs, and from the lungs, oxygen diffuses into the blood.
monoxide (CO) cannot. Carbon monoxide has a greater As soon as it enters the blood in enters the RBC is where
affinity for hemoglobin than oxygen. Therefore, when it binds to the hemoglobin. 3% of oxygen is dissolved in
carbon monoxide is present, it binds to hemoglobin the blood where is 97% of transported oxygen is bound to
preferentially over oxygen. As a result, oxygen cannot bind hemoglobin. This oxygen then diffuses in the tissues.
to hemoglobin, so very little oxygen is transported through Carbon dioxide is produced as a result of transpiration
the body (Figure 20.22). Carbon monoxide is a colorless, inside the body cells. from the cell the carbon dioxide
odorless gas and is therefore difficult to detect. It is diffuses into the blood where it is carried as:
produced by gas-powered vehicles and tools. Carbon 1. Carbaminohemoglobin, when it is bound to hemoglobin.
monoxide can cause headaches, confusion, and nausea; 2. Dissolved into the plasma.
long-term exposure can cause brain damage or death. 3. Bicarbonate ions.
Administering 100 percent (pure) oxygen is the usual carbon dioxide is maximally transported it as bicarbonate
treatment for carbon monoxide poisoning. Administration ions and it is taken back to the lungs with the help of
of pure oxygen speeds up the separation of carbon blood where it is released back into the atmosphere.
monoxide from hemoglobin. the diffusion of these gases takes place according to their
partial pressures.
What are the factors affecting the transport of gases?
Ans- Through the process of gaseous exchange, carbon
dioxide and oxygen move between the lungs and the
bloodstream. This is the main function of the respiratory
system which is required to make sure that a regular
supply of oxygen to tissues is required along with the
removal of carbon dioxide to check accumulation.
The main factors which affect the exchange of gases in
the lungs –
Figure 20.22. As percent CO increases, the oxygen ● The thickness of the membrane
saturation of hemoglobin decreases. ● The surface area of the membrane
● The difference in pressure across membranes
● Diffusion coefficient of the gas
You might also like
- Lung Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandLung Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Biology Presentation F4 8.3Document11 pagesBiology Presentation F4 8.3Michael AbishekapriyanNo ratings yet
- Lecture 7 (Gasses & Pressure)Document9 pagesLecture 7 (Gasses & Pressure)Άγγελος ΧαβέλαςNo ratings yet
- Introduction To Blood: PlasmaDocument7 pagesIntroduction To Blood: Plasmaعبدالحكيم النهديNo ratings yet
- Respiratory Physiology Part 2Document5 pagesRespiratory Physiology Part 2kabir musa ladanNo ratings yet
- 1.2 Transport of OxygenDocument6 pages1.2 Transport of OxygenDanielle HughesNo ratings yet
- Oxy Gas TransportationDocument6 pagesOxy Gas TransportationChemNo ratings yet
- Gaseous Exchange and RespirationDocument18 pagesGaseous Exchange and Respirationedain84No ratings yet
- Oxygenation BaseDocument2 pagesOxygenation BaseRehan ThomasNo ratings yet
- Adaptations of AlveoliDocument49 pagesAdaptations of AlveoliMoonNo ratings yet
- Oxygen and Carbon Dioxide Transport - PPTX JONELTADocument32 pagesOxygen and Carbon Dioxide Transport - PPTX JONELTArithikaNo ratings yet
- Anatomy & PhysiologyDocument3 pagesAnatomy & PhysiologyJohn Erlan LipataNo ratings yet
- How Red Blood Cells Transport Oxygen in the BodyDocument3 pagesHow Red Blood Cells Transport Oxygen in the BodySoha SonaNo ratings yet
- Human Respiratory System ExplainedDocument4 pagesHuman Respiratory System ExplainedSheehan MathurNo ratings yet
- The Respiratory System: Gas Exchange and ControlDocument38 pagesThe Respiratory System: Gas Exchange and Controlglenn johnstonNo ratings yet
- Abstrak: 1 - Fakultas Kedokteran UKRIDA - Blok 7: Respiratory 1Document9 pagesAbstrak: 1 - Fakultas Kedokteran UKRIDA - Blok 7: Respiratory 1TreitzNo ratings yet
- Fundamentals - CH 40: Oxygenation/Gas Exchange: Nloa Sfe B.8 Situation - Care of Client With Problems in OxygenationDocument20 pagesFundamentals - CH 40: Oxygenation/Gas Exchange: Nloa Sfe B.8 Situation - Care of Client With Problems in OxygenationSOLEIL LOUISE LACSON MARBASNo ratings yet
- B.8 Situation - Care of Client With Problems in OxygenationDocument11 pagesB.8 Situation - Care of Client With Problems in OxygenationSOLEIL LOUISE LACSON MARBASNo ratings yet
- Part 1: Critical Thinking: A. Arterial Blood Gas Analysis Is Commonly Used Diagnostic Tool To Evaluate The PartialDocument4 pagesPart 1: Critical Thinking: A. Arterial Blood Gas Analysis Is Commonly Used Diagnostic Tool To Evaluate The PartialJewel BerbanoNo ratings yet
- General Physiology II - Transport of Oxygen & Carbon - SIUST, College of DentistryDocument7 pagesGeneral Physiology II - Transport of Oxygen & Carbon - SIUST, College of DentistryNoor Al-Deen MaherNo ratings yet
- Educational ProgramsDocument28 pagesEducational ProgramsEsha KuttiNo ratings yet
- Maintaining healthy oxygenation through lifestyle choicesDocument12 pagesMaintaining healthy oxygenation through lifestyle choicesHasan A AsFourNo ratings yet
- Hb transports O2 & CO2Document31 pagesHb transports O2 & CO2sandramuganzaNo ratings yet
- Respiratory System Physiology PDFDocument121 pagesRespiratory System Physiology PDFVizhiNo ratings yet
- 11.17 Breathing and Exchanges of GasesDocument7 pages11.17 Breathing and Exchanges of GasesMoonesh MKNo ratings yet
- Altitude Physiology: PRESENTED BY Dick WilliamsDocument52 pagesAltitude Physiology: PRESENTED BY Dick WilliamsDeepak ArkalgudNo ratings yet
- SPM Notes On Chapter RespirationDocument18 pagesSPM Notes On Chapter RespirationSyamila YusofNo ratings yet
- 224392278 BMAT Biology Revision Notesรรร PDFDocument9 pages224392278 BMAT Biology Revision Notesรรร PDFB. ChillNo ratings yet
- Breathing & Exchange of GasesDocument5 pagesBreathing & Exchange of GasesMamta TiwariNo ratings yet
- Oxygen Transport from Lungs to CellsDocument6 pagesOxygen Transport from Lungs to CellsRahman Mukti AjiNo ratings yet
- Breathing and Exchange of GasesDocument5 pagesBreathing and Exchange of Gasessonu kumarNo ratings yet
- Diffusion, Blood Oxygen, Co2 EtcDocument13 pagesDiffusion, Blood Oxygen, Co2 EtcArun EbenezerNo ratings yet
- 11 Biology Notes ch17-pdf1Document9 pages11 Biology Notes ch17-pdf1LishaNo ratings yet
- 8.3 Gaseous Exchange in HumansDocument27 pages8.3 Gaseous Exchange in Humanswickedbiology101No ratings yet
- Reflection Respiratory SystemDocument4 pagesReflection Respiratory SystemAin Sufiza0% (1)
- Answer Discussion Share: (A) Inspiratory Reserve Volume (IRV) Expiratory Reserve Volume (ERV)Document8 pagesAnswer Discussion Share: (A) Inspiratory Reserve Volume (IRV) Expiratory Reserve Volume (ERV)gopeabhijit85No ratings yet
- The Physiology of Oxygen Delivery: DR Rob Law, DR H BukwirwaDocument4 pagesThe Physiology of Oxygen Delivery: DR Rob Law, DR H BukwirwaPrabhakar KumarNo ratings yet
- Human Resource ManagementDocument15 pagesHuman Resource ManagementKIPNGENO EMMANUELNo ratings yet
- Ribs Vertebral Column Sternum Abdominal Cavity Diaphragm Lungs Heart Blood Arteries VeinsDocument11 pagesRibs Vertebral Column Sternum Abdominal Cavity Diaphragm Lungs Heart Blood Arteries VeinsHamda HassanNo ratings yet
- 2.1 - Breathing and Gaseous ExchangeDocument16 pages2.1 - Breathing and Gaseous ExchangeZawata Afnan ShararaNo ratings yet
- List Major Muscles Involved in Respiration (Inspiration and Expiration) at Rest and During ExerciseDocument5 pagesList Major Muscles Involved in Respiration (Inspiration and Expiration) at Rest and During ExerciseEmMa DHieyahNo ratings yet
- Internal RespirationDocument9 pagesInternal RespirationRhose ReyesNo ratings yet
- Assignment Gaseous ExchangeDocument10 pagesAssignment Gaseous ExchangefetramyraNo ratings yet
- Factors Regulating BreathingDocument15 pagesFactors Regulating BreathingShubha DiwakarNo ratings yet
- Respiration Vs VentilationDocument1 pageRespiration Vs VentilationBoris FaktorovichNo ratings yet
- Ncert Solution Breathing and Exchange of GasesDocument10 pagesNcert Solution Breathing and Exchange of GasesAyush DuttaNo ratings yet
- Respiratory SystemDocument10 pagesRespiratory SystemSudip NeupaneNo ratings yet
- Function of The Respiratory SystemDocument5 pagesFunction of The Respiratory SystemRachelHatcher123No ratings yet
- Gaseous Exchange in Humans PDFDocument6 pagesGaseous Exchange in Humans PDFKevin JacksonNo ratings yet
- ODC Factsheet SummaryDocument3 pagesODC Factsheet Summarylastjoe71100% (1)
- Oxygenation Reflection PaperDocument2 pagesOxygenation Reflection PaperIRISH CACAYANNo ratings yet
- Oxygen InsufficiencyDocument24 pagesOxygen InsufficiencyRatna VimalNo ratings yet
- Patfis Critical Ill Focus On O2 DelivDocument10 pagesPatfis Critical Ill Focus On O2 Delivzidni fatayanNo ratings yet
- Science NotesDocument1 pageScience Notesshng0101No ratings yet
- The Respiratory SystemDocument3 pagesThe Respiratory SystemPiyush RoyNo ratings yet
- Breathing and Exchange of Gases: A Guide to RespirationDocument4 pagesBreathing and Exchange of Gases: A Guide to RespirationSreeyansu RajNo ratings yet
- Science Senior 2Document4 pagesScience Senior 2ajichristianbaniadamNo ratings yet
- CH 17Document20 pagesCH 17Maria BoccaNo ratings yet
- Lung Structure and Gaseous ExchangeDocument4 pagesLung Structure and Gaseous Exchangemakah2711No ratings yet
- Chapter 1: Respiration 1.1 The Human Breathing MechanismDocument4 pagesChapter 1: Respiration 1.1 The Human Breathing MechanismWindy SanNo ratings yet
- The Endocrine System: An OverviewDocument12 pagesThe Endocrine System: An Overviewlovelyc95No ratings yet
- Unit 2 Structure of Skeletal MuscleDocument2 pagesUnit 2 Structure of Skeletal MuscleMandeep DebnathNo ratings yet
- Unit 3 Respiratory PhysiologyDocument4 pagesUnit 3 Respiratory PhysiologyMandeep DebnathNo ratings yet
- Digestion and Absorption OverviewDocument3 pagesDigestion and Absorption OverviewMandeep DebnathNo ratings yet
- Unit 2 Structure of Neuron and Transmission of Nerve ImpulseDocument3 pagesUnit 2 Structure of Neuron and Transmission of Nerve ImpulseMandeep DebnathNo ratings yet
- Ecosystems as Complex SystemsDocument35 pagesEcosystems as Complex SystemsFelix Arthur Dioso100% (1)
- Cidam Earth and Life ScienceDocument16 pagesCidam Earth and Life ScienceEisle Keith Rivera Tapia100% (1)
- Pituitary Gland Functions and StructureDocument6 pagesPituitary Gland Functions and StructureaparnaNo ratings yet
- FUNGI SOMATIC STRUCTURES AND ASEXUAL REPRODUCTIONDocument36 pagesFUNGI SOMATIC STRUCTURES AND ASEXUAL REPRODUCTIONDevesh KumarNo ratings yet
- Temporalis Masseter Medial Pterygoid Lateral Pterygoid Muscles Temporal Fossa Infratemporal Fossa Mandible Temporomandibular JointDocument5 pagesTemporalis Masseter Medial Pterygoid Lateral Pterygoid Muscles Temporal Fossa Infratemporal Fossa Mandible Temporomandibular JointpmNo ratings yet
- Worksheet As Level Nucleic Acids and Protein Synthesis 1Document4 pagesWorksheet As Level Nucleic Acids and Protein Synthesis 1AreebNo ratings yet
- Russian ChernozemDocument439 pagesRussian ChernozemScary Creatures50% (2)
- Blastocystis Sp. To Treat or Not To Treat. 2012Document6 pagesBlastocystis Sp. To Treat or Not To Treat. 2012Nico RochaNo ratings yet
- B2.2 Movement in and Out of The CellDocument14 pagesB2.2 Movement in and Out of The Cellmenaga ilangkovanNo ratings yet
- Alpha Bio Tec Product-Catalog-2018 - MailDocument116 pagesAlpha Bio Tec Product-Catalog-2018 - MailMarin RabeiNo ratings yet
- Identificación AlternariaDocument42 pagesIdentificación AlternariaDiana BecerraNo ratings yet
- Grade-10-IM-Inventory - RODGEDocument10 pagesGrade-10-IM-Inventory - RODGEPaul AniceteNo ratings yet
- Malina 2020Document10 pagesMalina 2020Jaime VZNo ratings yet
- Aspek Laboratorium Untuk Menunjang Perencanaan Water Flooding Dan Water Flooding ImprovementDocument38 pagesAspek Laboratorium Untuk Menunjang Perencanaan Water Flooding Dan Water Flooding ImprovementInggit Yulias SaputriNo ratings yet
- Animal Diversity PearsonDocument55 pagesAnimal Diversity PearsonaidanajihahNo ratings yet
- Shahid 2009Document15 pagesShahid 2009Priyankan MajumderNo ratings yet
- Gibberelic Acid Regist. - Core GIBBER - 9-Section - 8Document5 pagesGibberelic Acid Regist. - Core GIBBER - 9-Section - 8Fered KhaliliNo ratings yet
- Arab Board Exam April 2015Document3 pagesArab Board Exam April 2015Firyal Balushi50% (4)
- PVA Nanoparticles for Protein DeliveryDocument10 pagesPVA Nanoparticles for Protein DeliverySandeep GargNo ratings yet
- CSIR NET Dec 2015 Ls Answer KeyDocument32 pagesCSIR NET Dec 2015 Ls Answer KeyAbhay Kumar50% (2)
- Ribosomes - Protein Construction Teams: RibosomeDocument2 pagesRibosomes - Protein Construction Teams: RibosomeIs SianNo ratings yet
- Food Science and NutritionDocument5 pagesFood Science and NutritionJostynaNo ratings yet
- BNMNS B370205Document8 pagesBNMNS B370205James PerianayagamNo ratings yet
- 04a Springer-Nature Ejournals Title-ListDocument57 pages04a Springer-Nature Ejournals Title-ListYaamini Rathinam K RNo ratings yet
- Goals and Fields of Psychology in 40 CharactersDocument45 pagesGoals and Fields of Psychology in 40 Charactersmuhammad umerNo ratings yet
- Corticosteroids Regulation and Clinical UsesDocument36 pagesCorticosteroids Regulation and Clinical UsesAbdur RafayNo ratings yet
- Wmsu Tos TemplateDocument29 pagesWmsu Tos TemplateHappy SasotaNo ratings yet
- Human Body Systems: 7 Grade ScienceDocument17 pagesHuman Body Systems: 7 Grade Sciencenona wayne dela peñaNo ratings yet
- Costa Et Al., 2008Document11 pagesCosta Et Al., 2008Angel LaraNo ratings yet
- Gen Bio - Immune SystemDocument28 pagesGen Bio - Immune SystemAvegel VillaciteNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet