Professional Documents
Culture Documents
Nota 6.1
Uploaded by
ONG JUN YAO MoeCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nota 6.1
Uploaded by
ONG JUN YAO MoeCopyright:
Available Formats
Chapter 6:Acids and alkalis
Acids
1. Acids are chemical compounds that containing
hydrogen which can replaced by a suitable metal.
2. Properties of acid:a)Tastes sour
b)Corrosive
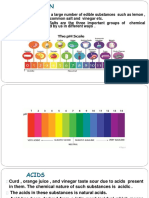
3.Has pH value less than 7.
4.Changes the colour of the blue litmus paper to red.
5.Reacts with metals like magnesium,aluminium,zinc
and iron to release hydrogen gas.
6.Acid can be divided into two types,they are organic
acid and inorganic acid.
Example of organic acids Source
Acetic acid Vinegar
Formic acid Ants&wasps
Lactic acid Sour milk
Malic acid Young apples
Citric acid Limes,oranges
Tannic acid Tea leaves
Tartaric acid Grapes
Example of inorganic Source
acids
Sulphuric acid Laboratories
Nitric acid Laboratories
Hydrochloric acid Laboratories
Carbonic acid Carbonated drinks
Alkali
1. Alkali is formed when oxide or metal hydroxide
dissolves in water.
2. Properties of alkali:
a)Tastes bitter
b)Feels slippery like the soap when touched
c) Corrosive
d)Has more than 7 pH value
e)Changes red litmus paper to blue
f)Does not react with metal to release hydrogen
gas
g)Reacts with salt ammonia produces salt,water
and release ammonia gas when heated.
Comparison between Acid and Alkali
Similarities
1)Both has corrosive characteristics.
2)Both show acidic and alkaline characteristics in
water.
Acid Differences Alkali
Sour Taste Bitter
No feel Feeling when Slippery
slippery touches
Changes to Effect on blue No change
red colour litmus paper
No change Effects on red Change to blue
litmus paper colour
Less than pH value Than7(pH8-14)
7(pH0-6)
Releases Reaction No reaction
hydrogen towards metal
Indicator Acid Neutral Alkaline
Litmus solution Red Purple Blue
Methyl Pink Orange Yellow
orange/red
Phenolphthalei Colourless Pale pink Bright
n red
Universal Yellow/red/ Green Blue
indicator orange violet/
purple
Source of acid in daily life:Car batteries,chemical
fertilisers,pickles,vinegar,paint,plastic,cakes,light
drinks,health salts,coagulate latex,Fruit juice,Food
preservative,Vitamin C pills,Clean metal
surfaces,Making fertilizers and explosives
Sources of Alkali in daily
life:Toothpaste,soap,medicine,slaked lime
fertiliser,cement,mortar,plastic and antacid medicine
成功的人靠的是成绩,失败的人找借口。
跟着蜜蜂你找蜂蜜,跟着苍蝇你找到厕所。
You might also like
- Nota 6.1Document5 pagesNota 6.1ONG JUN YAO MoeNo ratings yet
- 6.1 Properties of Acids and AlkalisDocument20 pages6.1 Properties of Acids and Alkalisxueer8993No ratings yet
- 6.1 Properties of Acids and AlkalisDocument20 pages6.1 Properties of Acids and Alkalismahfuzah sobriNo ratings yet
- 5.5 - Acid and AlkaliDocument12 pages5.5 - Acid and AlkaliKamal Dan AzmahNo ratings yet
- Acids and BasesDocument18 pagesAcids and Baseslin hassanNo ratings yet
- Science ProjectDocument7 pagesScience ProjectKenzy talkstoomuchNo ratings yet
- Acids Bases Salts Properties ReactionsDocument4 pagesAcids Bases Salts Properties ReactionsRounak BasuNo ratings yet
- Science Term 1 Acids and AlkalisDocument4 pagesScience Term 1 Acids and AlkalismikeNo ratings yet
- Presentation On ThemeDocument3 pagesPresentation On ThemeDee Anne MarieNo ratings yet
- Chapter 2 Acids Bases and SaltsDocument18 pagesChapter 2 Acids Bases and SaltsSatusha IndiaNo ratings yet
- Science Form 2: 5.5 Acid and AlkaliDocument38 pagesScience Form 2: 5.5 Acid and AlkalinurafziNo ratings yet
- Acids, Bases & Salts: A Guide to Common Household ChemicalsDocument7 pagesAcids, Bases & Salts: A Guide to Common Household ChemicalsRishi GovindaHarryNo ratings yet
- C11 Acids and BasesDocument54 pagesC11 Acids and BasesMyat Thura KyawNo ratings yet
- Acids and AlkalisDocument33 pagesAcids and AlkalisLubna ErumNo ratings yet
- Exam Study NotesDocument1 pageExam Study NotesfayzaansafiNo ratings yet
- Acids Bases Guide Properties Uses pH SafetyDocument2 pagesAcids Bases Guide Properties Uses pH SafetyFaith Joy Peñarejo QuiverNo ratings yet
- CBSE Class 10 Science Notes Chapter 2 Acids Bases and SaltsDocument19 pagesCBSE Class 10 Science Notes Chapter 2 Acids Bases and Saltsdrphysics256No ratings yet
- Chemistry: Acids, Bases and SaltsDocument8 pagesChemistry: Acids, Bases and SaltsNeeraj Poddar100% (1)
- Properties of Acids and Alkalis ExplainedDocument28 pagesProperties of Acids and Alkalis ExplainedNurul Husna50% (4)
- Acid and BaseDocument20 pagesAcid and BaseChris MaNo ratings yet
- Class 7 Science Notes Chapter - 5 Acids Bases and SaltsDocument5 pagesClass 7 Science Notes Chapter - 5 Acids Bases and SaltsKeerthan SureshNo ratings yet
- F2 Chapter 6 Acid and AlkaliDocument6 pagesF2 Chapter 6 Acid and AlkaliMei Shuen CheamNo ratings yet
- Chapter 6 - Acid and AlkaliDocument4 pagesChapter 6 - Acid and AlkaliDeacon ChiaNo ratings yet
- Chapter 2 Acids Bases and SaltsVer1Document18 pagesChapter 2 Acids Bases and SaltsVer1Vineet KhuranaNo ratings yet
- Understanding Acid and Alkaline PropertiesDocument27 pagesUnderstanding Acid and Alkaline PropertiesShahrul HisyamNo ratings yet
- Acids, Bases & Salts: Classification of IndicatorsDocument77 pagesAcids, Bases & Salts: Classification of IndicatorsreyanshNo ratings yet
- Chemistry - Chapter-5 Acids, Bases and SaltsDocument6 pagesChemistry - Chapter-5 Acids, Bases and SaltsShawty Got attitudeNo ratings yet
- Acid Base SaltDocument14 pagesAcid Base Saltshineblade99No ratings yet
- Acid Base and SaltsDocument16 pagesAcid Base and SaltsAnanya MishraNo ratings yet
- Grade 7-Notes On Acids Bases and SaltsDocument4 pagesGrade 7-Notes On Acids Bases and SaltsshamshadNo ratings yet
- Y7 T6.1 Acids and AlkaliDocument4 pagesY7 T6.1 Acids and Alkaligabriel phoonNo ratings yet
- Acids & Bases: They Are Everywhere.. in Your Food in Your House Even in You!!!!!Document12 pagesAcids & Bases: They Are Everywhere.. in Your Food in Your House Even in You!!!!!syrine mendozaNo ratings yet
- Class 7 Ch5 Notes AcidsDocument6 pagesClass 7 Ch5 Notes Acidsclass7science iisjNo ratings yet
- Acids, Bases and IndicatorsDocument12 pagesAcids, Bases and IndicatorsDavyieNo ratings yet
- Study Material For ChemistryDocument7 pagesStudy Material For ChemistryBHADRANo ratings yet
- Acids Bases and SaltsDocument55 pagesAcids Bases and Saltsgeorgy shibuNo ratings yet
- Form 2 Chapter 6 Acid and AlkaliDocument28 pagesForm 2 Chapter 6 Acid and AlkaliammyNo ratings yet
- Acids, Bases, & SaltsDocument55 pagesAcids, Bases, & SaltsShelley Chopra ChughNo ratings yet
- Acidsbases and Indicators - Chem - f1 - V1Document11 pagesAcidsbases and Indicators - Chem - f1 - V1Lubanga N JamesNo ratings yet
- Acid Base and SaltDocument15 pagesAcid Base and SaltMr. Sujan LamsalNo ratings yet
- Acid, Base and SaltsDocument21 pagesAcid, Base and SaltsPARTH WadheraNo ratings yet
- CH 10 Part 2Document7 pagesCH 10 Part 2Hend HamedNo ratings yet
- Ch12 Kitabcd Class 8 MSBHSE Science NotesDocument6 pagesCh12 Kitabcd Class 8 MSBHSE Science NotesONE CLICK COMPUTERNo ratings yet
- GR 7 NS WKSH 5 MemoDocument1 pageGR 7 NS WKSH 5 MemoPalesa KhumNo ratings yet
- IndicatorsDocument27 pagesIndicatorsKirithiga MuthusamyNo ratings yet
- Acids, Bases and PHDocument40 pagesAcids, Bases and PHdddsdsNo ratings yet
- Ch2 Rev NotesDocument17 pagesCh2 Rev NotesAshika Sai UnnikrishnanNo ratings yet
- Acids, Bases & OxidesDocument22 pagesAcids, Bases & OxidesMustafa ghazanfarNo ratings yet
- Acid and Base Worksheet 1Document2 pagesAcid and Base Worksheet 1letty100% (1)
- Acids, Bases and Salts Part 1 ExplainedDocument3 pagesAcids, Bases and Salts Part 1 ExplainedDhyan ShahNo ratings yet
- S2 Cfe Science Acids & Alkalis: High Acidity Low AcidityDocument4 pagesS2 Cfe Science Acids & Alkalis: High Acidity Low AcidityNevena GrujićNo ratings yet
- Acids, Bases and SaltsDocument31 pagesAcids, Bases and Saltssmi_santhoshNo ratings yet
- Acids & Bases: They Are Everywhere.. in Your Food in Your House Even in You!!!!!Document35 pagesAcids & Bases: They Are Everywhere.. in Your Food in Your House Even in You!!!!!crissaniaNo ratings yet
- Acids, Base and SaltsDocument3 pagesAcids, Base and SaltsGeorgia SimmsNo ratings yet
- Making a Homemade pH Indicator from Red Cabbage to Test Household SubstancesDocument7 pagesMaking a Homemade pH Indicator from Red Cabbage to Test Household SubstancesNikoli MajorNo ratings yet
- 5.5 Acids Alkali and NutralisationDocument13 pages5.5 Acids Alkali and NutralisationDayangNo ratings yet
- CSEC Chemistry - Acids, Bases and SaltsDocument4 pagesCSEC Chemistry - Acids, Bases and SaltsCornflakes ToastedNo ratings yet
- Acid, Base and Salt: by Iin Indriyati Biology Teacher of SMP 1 WonosariDocument23 pagesAcid, Base and Salt: by Iin Indriyati Biology Teacher of SMP 1 Wonosariivans augerNo ratings yet
- Acid and BasesDocument15 pagesAcid and BasesKunal HazarikaNo ratings yet
- Simple Probability (BM - I)Document6 pagesSimple Probability (BM - I)ONG JUN YAO MoeNo ratings yet
- 5.3 (A) Water Purification MethodDocument9 pages5.3 (A) Water Purification MethodONG JUN YAO MoeNo ratings yet
- 5.2 (C) Solubility and Rate of SolubilityDocument12 pages5.2 (C) Solubility and Rate of SolubilityONG JUN YAO MoeNo ratings yet
- 5.2 (B) Water As A Universal Solvent & Organic SolventDocument13 pages5.2 (B) Water As A Universal Solvent & Organic SolventONG JUN YAO MoeNo ratings yet
- Maintaining school safety and orderDocument2 pagesMaintaining school safety and orderONG JUN YAO MoeNo ratings yet
- Before LessonDocument4 pagesBefore LessonONG JUN YAO MoeNo ratings yet
- Acid-Base Neutralization Process ExplainedDocument3 pagesAcid-Base Neutralization Process ExplainedONG JUN YAO MoeNo ratings yet
- Book 1Document1 pageBook 1ONG JUN YAO MoeNo ratings yet
- After LessonDocument2 pagesAfter LessonONG JUN YAO MoeNo ratings yet
- Duty List Before LessonDocument10 pagesDuty List Before LessonONG JUN YAO MoeNo ratings yet
- C10 Sound WavesDocument3 pagesC10 Sound WavesONG JUN YAO MoeNo ratings yet
- A Leadership CampDocument1 pageA Leadership CampONG JUN YAO MoeNo ratings yet
- Report 13 06 17 06Document12 pagesReport 13 06 17 06ONG JUN YAO MoeNo ratings yet
- Report 27 6 1 7Document23 pagesReport 27 6 1 7ONG JUN YAO MoeNo ratings yet
- Afferent and Efferent Neurone DifferencesDocument2 pagesAfferent and Efferent Neurone DifferencesONG JUN YAO MoeNo ratings yet
- 2021 F1 Final RevisionDocument12 pages2021 F1 Final RevisionONG JUN YAO MoeNo ratings yet
- OxidesDocument27 pagesOxidesJuan KorNo ratings yet
- Effect of Alloying Elements On Steel PropertiesDocument2 pagesEffect of Alloying Elements On Steel PropertiesKARTHIGEYAN.RNo ratings yet
- 1.3 Formula and EquationsDocument44 pages1.3 Formula and EquationsDAVID ESCALANTE GILNo ratings yet
- Comp ChartDocument10 pagesComp ChartPriya KaleNo ratings yet
- 195 TOP Engineering Materials - Mechanical Engineering Multiple Choice Questions and Answers - MCQs Preparation For Engineering Competitive ExamsDocument20 pages195 TOP Engineering Materials - Mechanical Engineering Multiple Choice Questions and Answers - MCQs Preparation For Engineering Competitive Examssabya51278% (9)
- Analysis of Vegetables Fruit JuicesDocument4 pagesAnalysis of Vegetables Fruit Juices'Ashutosh' YadavNo ratings yet
- 6.9 Macronutrients and Micronutrients in PlantsDocument13 pages6.9 Macronutrients and Micronutrients in Plants143davbecNo ratings yet
- Diktat Naming Inorganic CompoundDocument6 pagesDiktat Naming Inorganic CompoundGeorge AthensNo ratings yet
- Lead Nitrate Chemistry Cbse 12 AnalysisDocument7 pagesLead Nitrate Chemistry Cbse 12 Analysis2066 Harini Manickam 12 C100% (1)
- Conceptual Physics - Chapter2Document29 pagesConceptual Physics - Chapter2Thursy SatrianiNo ratings yet
- Chemical Reactions Study Guide KeyDocument2 pagesChemical Reactions Study Guide KeyanyasastrenaNo ratings yet
- Leaching - Iron & SulfurDocument7 pagesLeaching - Iron & Sulfursudhu sudsNo ratings yet
- ExperimentDocument16 pagesExperimentcloudx chimNo ratings yet
- Experiment 8 - OrganohalidesDocument8 pagesExperiment 8 - OrganohalidesOrlando Angelo CerezoNo ratings yet
- Acids Bases and Salts For Grade 7Document36 pagesAcids Bases and Salts For Grade 7raynjeremay100% (1)
- PUREX Process Configuration and Advanced Reprocessing MethodsDocument4 pagesPUREX Process Configuration and Advanced Reprocessing Methodshenry leeNo ratings yet
- Third Long Test Grade 8 Science: Gen. Ricardo Papa Sr. Memorial High SchoolDocument2 pagesThird Long Test Grade 8 Science: Gen. Ricardo Papa Sr. Memorial High SchoolEunice CorreaNo ratings yet
- Applications of Organometallic CompoundsDocument9 pagesApplications of Organometallic CompoundsUsama Talib100% (1)
- QueDocument29 pagesQuedeva1007No ratings yet
- TheSecretofHydrogenRichWater Hidemitsu HayashiDocument3 pagesTheSecretofHydrogenRichWater Hidemitsu Hayashiiakhan45100% (1)
- Classification of Inorganic Polymers: Dr. PriyankaDocument26 pagesClassification of Inorganic Polymers: Dr. PriyankaTanu SinghNo ratings yet
- CN 15 en 05Document19 pagesCN 15 en 05Toni D.No ratings yet
- Ap ChemDocument4 pagesAp ChemEthan NguyenNo ratings yet
- Chemistry Paper 1 2020 Higher TierDocument32 pagesChemistry Paper 1 2020 Higher Tiercheez denchNo ratings yet
- Lanthanides ExtractionDocument11 pagesLanthanides Extractionibrahim ali elalfy100% (4)
- Wilder GetterDocument13 pagesWilder GetterFerhat Bozduman100% (1)
- 2 Manufacture of Ammonia, Nitric Acid and Calcium Ammonium NitrateDocument14 pages2 Manufacture of Ammonia, Nitric Acid and Calcium Ammonium NitrateKarez MartoNo ratings yet
- Copper Nickel AlloysDocument5 pagesCopper Nickel AlloysAditya Agarwal100% (2)
- Activity Sheets For Chem With NamesDocument7 pagesActivity Sheets For Chem With NamesJoan PenanoNo ratings yet