Professional Documents
Culture Documents
Boyle's and Charles' Law
Uploaded by
Carl Lester RapaconOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Boyle's and Charles' Law
Uploaded by
Carl Lester RapaconCopyright:
Available Formats
Republic of the Philippines
UNIVERSITY OF NORTHERN PHILIPPINES
Tamag, Vigan City
2700 Ilocos Sur
Laboratory Schools
Science 10
Name: Neil Bert R. Rapacon Date: 4 – 24 - 2022
A. Concept Application-Situational Analysis
Complete the table below by analyzing first the situation before supplying
answers to the blank boxes.
Situation Is it an application on Explain your answer
Boyle’s Law or Charles’s
Law?
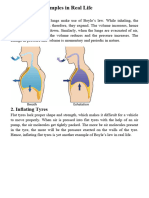
1. Breathing While inhaling the pressure
decreasing and the volume
Boyle’s Law is increasing. It is vice
versa, while exhaling your
pressure increases and the
volume is decreasing.
2. Placing a dented ping pong Temperature is responsible
ball in a hot water. for this situation. The
dented ping pong ball will
remain dented in cold
Charles’ Law
surroundings, and it will
expand in hot surroundings.
The reason behind this is
gas is fast and free moving
in hot surfaces.
3. Flying a hot air balloon. Again, temperature is a
factor here. As the flames of
Charles’ Law the hot air balloon is more
bigger and hotter, the
volume will also increase
and expand.
4. Pulling up the plunger of a The same principle, as the
syringe. plunger of the syringe pulls
up the pressure will also
pull up and decreases which
it will help to increase the
amount of volume inside the
syringe. Again vice versa, if
Boyle’s Law you push down the plunger
of the syringe the pressure
will also push down, and it
will increase which it will
help to decrease the amount
of volume.
5. Buying a helium balloon Helium is sensitive to
early morning instead of temperatures. In morning,
afternoon. we all know that
temperature is low than
afternoon. The hotness and
coldness of the surrounding
will affect the helium gas
inside the balloon. For
Charles law example, the helium-based
balloon will shrink in
morning due to coldness,
while it will expand and
possibly burst because of
hotness
B. Concept Application-Complete Me
Choose the correct word inside the parenthesis. Write your answer before the letter.
1. A tank (not rigid) contains 2.4 L of helium gas at 25℃. What will be the volume of the
tank after heating it and its content to 41℃ temperature at constant pressure?
Charles’ Law a. This is an application of (Boyle’s Law, Charles’ Law).
Volume b. The unknown quantity in the problem is the (volume, temperature,
pressure) of the gas.
V2 𝑇1 = V1𝑇2 c. The formula needed to solve the problem is
(𝑃1𝑉1 = 𝑃2𝑉2, V2 𝑇1 = V1𝑇2).
Increases d. The temperature of the gas (decreases, increases).
Increases e. The volume of the gas (decreases, increases).
2. A sample of fluorine gas occupies a volume of 510 mL at 760 mmHg. If the temperature
remains the same, calculate the pressure required to reduce its volume to 170 mL.
Boyle’s Law a. This is an application of (Boyle’s Law, Charles’ Law).
Pressure b. The missing quantity in the problem is the (volume,
temperature, pressure) of the gas.
𝑃1𝑉1 = 𝑃2𝑉2 c. The formula needed to solve the problem is
(𝑃1𝑉1 = 𝑃2𝑉2, 𝑇1 = 𝑇2).
Decreases d. The volume of the gas (decreases, increases).
Increases e. The pressure of the gas (decreases, increases).
You might also like
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Emerald Green and Gold Elegant Applicant Cover LetterDocument4 pagesEmerald Green and Gold Elegant Applicant Cover LetterSam Yvhanne Quinzon TanNo ratings yet
- LEARNING ACTIVITY SHEET 5 (Charles' Law)Document2 pagesLEARNING ACTIVITY SHEET 5 (Charles' Law)cherrymaeregalario2001No ratings yet
- Charles' Law..Document7 pagesCharles' Law..Aira Villarin100% (2)
- DHEA CAPUYAN - Charles's Law Science ExperimentDocument4 pagesDHEA CAPUYAN - Charles's Law Science ExperimentDhea Angela A. CapuyanNo ratings yet
- Miguel, John Ryan, D.G Lab Act 7Document3 pagesMiguel, John Ryan, D.G Lab Act 7Simon DeleonNo ratings yet
- GeneralChemistry 1 W7 Gaga AMaryCarmelDocument5 pagesGeneralChemistry 1 W7 Gaga AMaryCarmelEloisa MadrilenoNo ratings yet
- Mero, Lei Anne Jaye V. 10-ST. JAMES 10D49Document1 pageMero, Lei Anne Jaye V. 10-ST. JAMES 10D49Lei Anne MeroNo ratings yet
- 3-4 Gas Laws Int - Reader - Study - Guide PDFDocument6 pages3-4 Gas Laws Int - Reader - Study - Guide PDFVara BikkinaNo ratings yet
- Science 10 - Q4 - Week 1 - Day 4Document3 pagesScience 10 - Q4 - Week 1 - Day 4Archie CabaNo ratings yet
- Detailed Lesson PlanDocument2 pagesDetailed Lesson PlanErica Mae AlcaldezaNo ratings yet
- Charles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayDocument2 pagesCharles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayEnriquez JohnNo ratings yet
- DLP 2 (AutoRecovered)Document4 pagesDLP 2 (AutoRecovered)Luz DaceraNo ratings yet
- Document About Literature and HistoryDocument2 pagesDocument About Literature and HistoryMarieta GonzalesNo ratings yet
- Q4 Science 10 Week2Document3 pagesQ4 Science 10 Week2Edison Caringal50% (2)
- Lesson Plan About Gay Lussacs Law 1Document10 pagesLesson Plan About Gay Lussacs Law 1Lyca Mae De Villa100% (2)
- Enriquez - CJ Chapter 3Document23 pagesEnriquez - CJ Chapter 3Sir JeNo ratings yet
- Experiment 3 GasesDocument8 pagesExperiment 3 GasesNathanael Kean DimasacatNo ratings yet
- Grade VII-Physics - L4 Heat WB SolutionDocument4 pagesGrade VII-Physics - L4 Heat WB SolutionDEBENDRA NATH CHOUDHURYNo ratings yet
- Valle - Q4W1 SciDocument6 pagesValle - Q4W1 SciAlthea Camilla ValleNo ratings yet
- Detailed Lesson Plan (DLP) Format: Instructional PlanningDocument3 pagesDetailed Lesson Plan (DLP) Format: Instructional PlanningRitz Anton LimNo ratings yet
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- S10MT Iva B 21 KINETIC MOLECULAR THEORY ABUEVADocument5 pagesS10MT Iva B 21 KINETIC MOLECULAR THEORY ABUEVALeil Riego0% (1)
- Genchem Experiments Gas LawsDocument7 pagesGenchem Experiments Gas LawsMr JNo ratings yet
- CL FridayDocument4 pagesCL FridayjeshellabendiciogulbinNo ratings yet
- Santiago City Tel/Fax: (078) - 682-8454 / 305-0957 WWW - Northeasterncollege.edu - PHDocument8 pagesSantiago City Tel/Fax: (078) - 682-8454 / 305-0957 WWW - Northeasterncollege.edu - PHjeffersonNo ratings yet
- Detailed-LP - Grade 8Document9 pagesDetailed-LP - Grade 8Peter Dave Moralda PedrosoNo ratings yet
- Boyles Law Lesson PlanDocument2 pagesBoyles Law Lesson PlanFany Fabia60% (5)
- Intermolecular Forces Properties of Liquid Stem 2Document39 pagesIntermolecular Forces Properties of Liquid Stem 2nolimayolivaNo ratings yet
- DLP Grade 10 ScienceDocument13 pagesDLP Grade 10 ScienceAnne SajulNo ratings yet
- Charles Law DLPDocument9 pagesCharles Law DLPRINA MORENONo ratings yet
- Revised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarDocument11 pagesRevised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarSaysain UkayNo ratings yet
- Thermody Lab About Egg Chuchu PDFDocument4 pagesThermody Lab About Egg Chuchu PDFGievel Enoroba LopezNo ratings yet
- Practical-1 (Demonstration of Basic Physics in CompDocument3 pagesPractical-1 (Demonstration of Basic Physics in CompHassan Iftekhar AhmedNo ratings yet
- Chap 13Document34 pagesChap 13Iván MartinezNo ratings yet
- Tilles, Mara - Science - JHSDocument3 pagesTilles, Mara - Science - JHSMara TillesNo ratings yet
- Leap-G7 Q3 W6 Heat-Transfer Ongkiko-Marilyn Sdo-DasmarinasDocument4 pagesLeap-G7 Q3 W6 Heat-Transfer Ongkiko-Marilyn Sdo-DasmarinasMary Grace Sambayan LlanesNo ratings yet
- LAS 1 Gas Laws - Charles' LawDocument3 pagesLAS 1 Gas Laws - Charles' LawSalve Serrano100% (1)
- Share GEN-CHEM-Q4 - LP2Document8 pagesShare GEN-CHEM-Q4 - LP2Jenny Manzanillo MirabonaNo ratings yet
- Chem Law LawDocument2 pagesChem Law LawFaye Lianne MaderoNo ratings yet
- Dipping Bird - Parker BaxterDocument2 pagesDipping Bird - Parker BaxterparkerbaxterNo ratings yet
- Physics Chapter 9 (Chel, NIa, Sal)Document28 pagesPhysics Chapter 9 (Chel, NIa, Sal)salma salNo ratings yet
- Week 1 Day 3Document7 pagesWeek 1 Day 3jakeblas07No ratings yet
- DLL Week 7 FontillasDocument4 pagesDLL Week 7 Fontillasbren.abadNo ratings yet
- Charles Law. NewDocument5 pagesCharles Law. NewMarvin Agustin100% (1)
- Tilles, Mara - Science - JHSDocument3 pagesTilles, Mara - Science - JHSMara TillesNo ratings yet
- Behaviour of Gases (Boyles Law LPDocument8 pagesBehaviour of Gases (Boyles Law LPOmhar CeresNo ratings yet
- Conceptual FrameworkDocument1 pageConceptual FrameworkoutcohldNo ratings yet
- Charles LawDocument12 pagesCharles LawLerr Real RelleNo ratings yet
- 4 Cooling Techniques Handout PDFDocument25 pages4 Cooling Techniques Handout PDFSergio PastorNo ratings yet
- GAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidDocument9 pagesGAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidMichiiee BatallaNo ratings yet
- Gas Law in Deep-Sea Diving: Boyles & Charles's LawsDocument7 pagesGas Law in Deep-Sea Diving: Boyles & Charles's LawsShanie Rose A. HermocillaNo ratings yet
- What Is Air Track?: 2.1.1 Buoyancy or Archimedes' PrincipleDocument6 pagesWhat Is Air Track?: 2.1.1 Buoyancy or Archimedes' PrinciplekktayNo ratings yet
- Boyle's Law LESSON PLANDocument5 pagesBoyle's Law LESSON PLANQueencess Ara TorresNo ratings yet
- PHYSICS Chapter21 - Temperature, Heat and Expansion PDFDocument24 pagesPHYSICS Chapter21 - Temperature, Heat and Expansion PDFAanya gupta100% (1)
- If You Play-WPS OfficeDocument1 pageIf You Play-WPS Officeawitg031No ratings yet
- Grade 10fourth Quarter: Boyle's LawDocument1 pageGrade 10fourth Quarter: Boyle's LawRommer PublicoNo ratings yet
- G10 SCI Q4WK1 LAS1-checked-and-editedDocument5 pagesG10 SCI Q4WK1 LAS1-checked-and-editedKirsten Tiffany TriasNo ratings yet
- Heattempphases 1 HspsDocument12 pagesHeattempphases 1 Hspsapi-325864985No ratings yet
- The Energy That Warms Us: A Look at HeatFrom EverandThe Energy That Warms Us: A Look at HeatRating: 1 out of 5 stars1/5 (1)
- thống số cụm phanh sau 777E KYDDocument2 pagesthống số cụm phanh sau 777E KYDlongcpqn95No ratings yet
- Gear BoxDocument36 pagesGear BoxNimoNo ratings yet
- Solutions and Solubility 2021Document3 pagesSolutions and Solubility 2021Mauro De LollisNo ratings yet
- 2003044rev1 PDFDocument2 pages2003044rev1 PDFMML LTDNo ratings yet
- Guidelines On Water ProofingDocument88 pagesGuidelines On Water ProofingViệt Đặng XuânNo ratings yet
- Inertia FormulasDocument4 pagesInertia FormulasLoysa Agtarap MataNo ratings yet
- Brochure - Digilogic Systems PVT Ltd.Document28 pagesBrochure - Digilogic Systems PVT Ltd.ManojGRamakrishnaNo ratings yet
- Advanced MultisimDocument146 pagesAdvanced MultisimHec Itou75% (4)
- Biomedical Short Learning Article CompleteDocument169 pagesBiomedical Short Learning Article Completenebiyu mulugetaNo ratings yet
- Leaf Spring - Final DocumentationDocument64 pagesLeaf Spring - Final DocumentationSushmitha VaditheNo ratings yet
- AQA GCSE Chemistry: 2.1.5 Metallic BondingDocument1 pageAQA GCSE Chemistry: 2.1.5 Metallic BondingZehmilNo ratings yet
- When Bad Things Happen To Good MissilesDocument9 pagesWhen Bad Things Happen To Good Missilesmykingboody2156No ratings yet
- Interview Q SASDocument43 pagesInterview Q SAShimaNo ratings yet
- Exemplar Gr1 Maths Diagnostic Assessment - Term 2 - 2021Document4 pagesExemplar Gr1 Maths Diagnostic Assessment - Term 2 - 2021Liandra'Lulu'VdMerweNo ratings yet
- Juniper SRX Quickstart-12.1r3Document455 pagesJuniper SRX Quickstart-12.1r3Pichai Ng-arnpairojhNo ratings yet
- Unit QuestionsDocument155 pagesUnit QuestionsSanya KhanNo ratings yet
- Chemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsDocument47 pagesChemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsNguyễn Quốc HưngNo ratings yet
- Design Data (No Longer Current But Cited in The Building Regulations)Document290 pagesDesign Data (No Longer Current But Cited in The Building Regulations)ferdinand bataraNo ratings yet
- Open-Air Theatre in The Centre of The City: Acoustic Design and Noise Environment ControlDocument3 pagesOpen-Air Theatre in The Centre of The City: Acoustic Design and Noise Environment ControlArchitectural Visualizations ArchitecturelandsNo ratings yet
- SMAC Actuators User ManualDocument52 pagesSMAC Actuators User ManualGabo DuarNo ratings yet
- Lutron / Trane Bacnet Integration: QuantumDocument7 pagesLutron / Trane Bacnet Integration: QuantumthomasNo ratings yet
- 10th Class-Maths Text Book-NewDocument400 pages10th Class-Maths Text Book-NewVishnu Muddasani100% (1)
- Conic SectionDocument9 pagesConic SectionJomana MacalnasNo ratings yet
- Quarter 1 Week 1Document6 pagesQuarter 1 Week 1GhghaaaNo ratings yet
- Cad and Dog 2Document5 pagesCad and Dog 2Muhammad RifaiNo ratings yet
- ACUCOBOL-GT Users Guide v8 Tcm21-18390Document587 pagesACUCOBOL-GT Users Guide v8 Tcm21-18390Miquel CerezuelaNo ratings yet
- Ec1102n - Mimi Nur Nabila Binti Alias - 2019293128 - Final ExamDocument17 pagesEc1102n - Mimi Nur Nabila Binti Alias - 2019293128 - Final ExammimiNo ratings yet
- Fractional Order Derivative and Integral Using LabVIEWDocument13 pagesFractional Order Derivative and Integral Using LabVIEWauraliusNo ratings yet
- Windy Hill Middle School - Trumpet Warm Up BookDocument61 pagesWindy Hill Middle School - Trumpet Warm Up BookGleyce VieiraNo ratings yet
- Binocular Vision Development, Depth Perception and DisordersDocument275 pagesBinocular Vision Development, Depth Perception and DisordersJuliana ToméNo ratings yet