Professional Documents
Culture Documents
Solids in Liquids - Solubility and Miscibility
Uploaded by
Baini JamalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solids in Liquids - Solubility and Miscibility
Uploaded by
Baini JamalCopyright:
Available Formats
Result:
A. Solids In Liquids
Solute
Solvent
Sodium Chloride Oxalic Acid Paraffin
Water Soluble Not Completely Not Dissolve
Dissolve
Ethanol Not Dissolve Not Dissolve Not Dissolve
Hexane Crystalline Solid Is Insoluble Dissolve
Formed
Table 1
B. Miscibility Of Liquids
Solvent Observations
Water Immiscibility. Oil formed a layer above water
Ethanol Partially miscible. Mixture turned cloudy and not completely dissolve
Hexane Miscible. A clear mixture.
Table 2
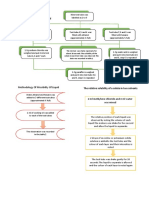
C. The Relative Solubility of a Solute in Two Solvents
1. Observation: Methylene chloride formed a layer below water because it is not miscible with water.
Water has lower density than methylene chloride and opposite to it.
2. Colour of the aqueous layer: Turn yellowish
Colour of the methylene chloride layer: Clear
3. Colour of the aqueous layer: Still yellowish
Colour of the methylene chloride layer: Turn dark pink
4. Based on the relative intensity of the colour of the two layer, iodine dissolve more in methylene
chloride than water since the colour of pure iodine is dark pink. Thus, iodine properties dissolve
fully in methylene chloride.
You might also like
- Print Expt7 Lab ReportDocument7 pagesPrint Expt7 Lab ReportShaliza Hernandez100% (2)
- The Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksFrom EverandThe Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksNo ratings yet
- Bullies, Socialites and the Shy Ones: Exploring the Personalities of Watercolor PaintsFrom EverandBullies, Socialites and the Shy Ones: Exploring the Personalities of Watercolor PaintsNo ratings yet
- Experiment 13 (Syntheses of Soap and Detergent)Document5 pagesExperiment 13 (Syntheses of Soap and Detergent)Cheng Bauzon100% (1)
- Biochem Lab Activity 5Document47 pagesBiochem Lab Activity 5Nafeesa Cadir100% (1)
- Introductory Chemistry For Today 8Th Edition Seager Solutions Manual Full Chapter PDFDocument36 pagesIntroductory Chemistry For Today 8Th Edition Seager Solutions Manual Full Chapter PDFcara.miltner626100% (11)
- Introductory Chemistry For Today 8th Edition Seager Solutions Manual 1Document34 pagesIntroductory Chemistry For Today 8th Edition Seager Solutions Manual 1shantel100% (45)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Solutions Manual Full Chapter PDFDocument36 pagesChemistry For Today General Organic and Biochemistry Hybrid Edition 8Th Edition Seager Solutions Manual Full Chapter PDFelise.green301100% (11)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions Manual 1Document36 pagesChemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions Manual 1charleslopezqxstcfgbka100% (26)
- Chemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions Manual 1Document34 pagesChemistry For Today General Organic and Biochemistry Hybrid Edition 8th Edition Seager Solutions Manual 1shirley100% (37)
- Ex 1. Solubility As Physical PropertyDocument7 pagesEx 1. Solubility As Physical PropertyGlister Diadem DolleraNo ratings yet
- Kate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 11 Acyl Compounds: Soaps and DetergentsDocument5 pagesKate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 11 Acyl Compounds: Soaps and DetergentsKateNo ratings yet
- Lab Report8Document5 pagesLab Report8wanjiaNo ratings yet
- Kate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 12 Amines, Amino Acids and ProteinsDocument9 pagesKate Coleen D. Galera BS in Chemistry II May 4, 2017 Experiment 12 Amines, Amino Acids and ProteinsKateNo ratings yet
- Aim: To Understand The Different Types of Solutions and To Examine The DifferentDocument3 pagesAim: To Understand The Different Types of Solutions and To Examine The DifferentKeenanNo ratings yet
- SCIence 7 Investigates at and UnsatDocument11 pagesSCIence 7 Investigates at and UnsatbrgyNo ratings yet
- Functional Group Analysis 1Document17 pagesFunctional Group Analysis 1حسين أحمد حسينNo ratings yet
- BiochemistryDocument2 pagesBiochemistryvicerra85No ratings yet
- Xiao 101083582 Report1Document6 pagesXiao 101083582 Report1zhiqiaoxiao7No ratings yet
- Properties Methanol Phenol N-Butyl Sec-Butyl Tert-Butyl: Experiment 10 Alcohols and Phenols Data and ResultsDocument2 pagesProperties Methanol Phenol N-Butyl Sec-Butyl Tert-Butyl: Experiment 10 Alcohols and Phenols Data and ResultsPrincess Loyola TapiaNo ratings yet
- Systematic Organic AnalysisDocument6 pagesSystematic Organic Analysisapi-19520338100% (5)
- General Chemistry Performance in a NutshellDocument4 pagesGeneral Chemistry Performance in a NutshellRoxane RosendoNo ratings yet
- SolutionsDocument49 pagesSolutionsPeter Jeff LauretaNo ratings yet
- Preparation and Identification of Sodium AcetateDocument2 pagesPreparation and Identification of Sodium AcetateDarlene Faith BeroñaNo ratings yet
- Chem II Solutions ActivityDocument1 pageChem II Solutions ActivityAmee KiNo ratings yet
- Solubility NatureDocument5 pagesSolubility NatureDej IdleNo ratings yet
- Expt8 - Fats and Oils and Soaps and Detergents - Answersheet (2) - SecaDocument3 pagesExpt8 - Fats and Oils and Soaps and Detergents - Answersheet (2) - SecaElleeze Gwyneth EmpialesNo ratings yet
- Lipids Activity 9 - Characterization Tests of Lipids Including Cottonseed Oil, Coconut Oil, CholesterolDocument25 pagesLipids Activity 9 - Characterization Tests of Lipids Including Cottonseed Oil, Coconut Oil, CholesterolJULIANNAH ATHENA MERCADONo ratings yet
- Experiment 8: Properties of Organic Compounds With Carbonyl GroupDocument7 pagesExperiment 8: Properties of Organic Compounds With Carbonyl GroupMarita AlcansadoNo ratings yet
- Mixture: SolutionDocument7 pagesMixture: SolutionAli RafaatNo ratings yet
- Physical Properties of SolutionsDocument38 pagesPhysical Properties of SolutionsAntonio Exal ColladoNo ratings yet
- Experiment No. 8 Fats and Oils: Soaps and Detergents I. DataDocument4 pagesExperiment No. 8 Fats and Oils: Soaps and Detergents I. DataKleya ParreñoNo ratings yet
- ORGANIC CHEMISTRY REACTIONSDocument7 pagesORGANIC CHEMISTRY REACTIONSASYRANI ZULAIKHANo ratings yet
- Chemistry Book - Experimental TechniquesDocument10 pagesChemistry Book - Experimental TechniquesAgustina RIVERO SEGURANo ratings yet
- Partial DataDocument2 pagesPartial DataKarla CeaNo ratings yet
- Chem 120.1 Laboratory Report No. 7Document4 pagesChem 120.1 Laboratory Report No. 7JM BoylesNo ratings yet
- Pertemuan 7 - Larutan, Solute, Solven, SolubilityDocument51 pagesPertemuan 7 - Larutan, Solute, Solven, SolubilityNing CahNo ratings yet
- EsterificationDocument13 pagesEsterificationAkshay bhuranNo ratings yet
- Chem 20 Lab Experiment 8 Fats and Oils Soaps and DetergentsDocument4 pagesChem 20 Lab Experiment 8 Fats and Oils Soaps and DetergentsChristine MarcellanaNo ratings yet
- Answer Sheet For Activity 8Document9 pagesAnswer Sheet For Activity 8RHEA ANGELICA ATILANO GREGORIONo ratings yet
- Hydrocarbon Solubility and ReactionsDocument10 pagesHydrocarbon Solubility and ReactionsKateNo ratings yet
- 5350-5354 Reference Tables - Description and Solubility - ADocument5 pages5350-5354 Reference Tables - Description and Solubility - Apate malabananNo ratings yet
- Mixtures and Solutions - StudentsDocument6 pagesMixtures and Solutions - Studentschadling.jim23No ratings yet
- Carbonyl Compounds and Carbohydrates I.Data and Results Compound Homogeneity ObservationsDocument11 pagesCarbonyl Compounds and Carbohydrates I.Data and Results Compound Homogeneity ObservationsKateNo ratings yet
- Solubility Principles and Applications in PharmacyDocument27 pagesSolubility Principles and Applications in PharmacyPuspa DasNo ratings yet
- Solubility Experiment at HomeDocument12 pagesSolubility Experiment at HomeJohn Joseph YeeNo ratings yet
- G.6 Q.1 SCIENCE Lesson 2 Homogeneous MixtureDocument35 pagesG.6 Q.1 SCIENCE Lesson 2 Homogeneous MixturemeguiNo ratings yet
- Chapter 12-SolutionsDocument32 pagesChapter 12-SolutionsNada MeselhyNo ratings yet
- 3. PROPERTIES OF SOLUTIONDocument20 pages3. PROPERTIES OF SOLUTIONApril Rose DeoronioNo ratings yet
- SolutionsDocument47 pagesSolutionsblismae genotivaNo ratings yet
- Quiz Discussion Organic Chemistry Act.Document4 pagesQuiz Discussion Organic Chemistry Act.quirenicoleNo ratings yet
- Investigating Solubility and Acid-Base ReactionsDocument11 pagesInvestigating Solubility and Acid-Base ReactionsJackie MolstadNo ratings yet
- EXPE9Document8 pagesEXPE9K-yanVehraaYomomaNo ratings yet
- Experiment 4:5 Lab ReportDocument6 pagesExperiment 4:5 Lab ReportHannah GonzalesNo ratings yet
- Xt202000826@wmsu@edu - PH: Activity No. 5 Reaction of LipidsDocument5 pagesXt202000826@wmsu@edu - PH: Activity No. 5 Reaction of LipidsJohanna Marie GantalaoNo ratings yet
- Mixtures GuideDocument5 pagesMixtures GuideSusan BrowneNo ratings yet
- Methodology of Solubility and Miscibility (Lab 1)Document1 pageMethodology of Solubility and Miscibility (Lab 1)Baini JamalNo ratings yet
- Result For Buffer Lab 2Document1 pageResult For Buffer Lab 2Baini JamalNo ratings yet
- Buffer SlideDocument21 pagesBuffer SlideBaini JamalNo ratings yet
- Buffer Solution Methodology and Buffering Action DeterminationDocument1 pageBuffer Solution Methodology and Buffering Action DeterminationBaini JamalNo ratings yet