Professional Documents
Culture Documents
Format Paper
Format Paper
Uploaded by
Umar ZulfiqarOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Format Paper
Format Paper
Uploaded by
Umar ZulfiqarCopyright:
Available Formats
PUNJAB COLLEGES HAFIZABAD
Time: FORTNIGHTLY TEST (B) Marks: 30
Subject: Inter Part-I
Name: _______________________Roll No: ________ Section: _________
OBJECTIVE TYPE
Note: You have four choices for each objective type question as A, B, C, and D. The choice which you
think is correct; encircle it. Use marker or pen to encircle. Cutting or overwriting will result in zero
mark in that question.
Q.1 Encircle the correct Option. 06
1. One mole of SO2 contains oxygen atoms.
A. 6.02 x 1023 B. 12.04 x 1023 C. 1 mole D. 3 mole
2. Number of atoms of phosphorous in one mole of AlPO4
A. 6.02 x 1023 B. 3 x 6.02 x 1023 C. 0.5 x 6.02 x 1023 D. 4 x 6.02 x 1023
3. 1.12dm3 of O2 gas at any condition has mass of O2 gas.
A. 1.6 g B. 16 g C. 32 g D. None
4. The ratio of actual yield to theoretical yield multiplied by 100 is called:
A. Complex yield B. Experimental yield C. Percentage yield D. None
5. The correct relationship is
A. 1 psi = 6.803 x 10 -2 atm C. 14.7 Psi = 101325 Nm -2
B. 1 atm = 76 cm of Hg D. All of these
6. Mathematically Boyle’s law is shown as:
A. PT = K B. VT = K C. P/T = K D. PV = K
SUBJECTIVE TYPE
SECTION-I
Note: All the questions in subjective type are compulsory.
Q.2 Give short answers of the followings. 16
i. Define Avogadro’s number. Give its numerical value.
ii. Write down limitations of a chemical equation.
iii. List steps involved to identity a limiting reactant?
iv. Define stoichiometry. Give two assumptions for stoichiometric calculations.
v. How percentage yield is calculated?
vi. Differentiate between actual yield and theoretical yield?
vii. Define Boyle’s law and give its mathematical expression?
viii. Define Atmospheric pressure. Give its two units.
SECTION-II 08
Q.3 (a) When limestone (CaCO3) is roasted, quicklime (CaO) is produced according to the following
reaction.
CaCO3 CaO + CO2
The actual yield of CaO is 2.5 kg, when 4.5 kg of limestone is roasted. What is the percentage yield of this
reaction?
(b) Give graphical explanation of Boyle’s Law.
You might also like
- Addis Ababa City Government Education BureauDocument11 pagesAddis Ababa City Government Education BureauErmias100% (1)
- First Periodical Exam Chemistry 2Document10 pagesFirst Periodical Exam Chemistry 2Rogelio PontejoNo ratings yet
- Htri Presentation PDFDocument53 pagesHtri Presentation PDFanghel_florin82No ratings yet
- Quarter 1 - General Chemistry 1Document11 pagesQuarter 1 - General Chemistry 1garry100% (3)
- Chemistry EUEE From 2000-2011Document53 pagesChemistry EUEE From 2000-2011Yonilo memeloNo ratings yet
- Physical Science 2019Document5 pagesPhysical Science 2019L Lawliet100% (1)
- General Chemistry 1 Module Week 1 and 2Document67 pagesGeneral Chemistry 1 Module Week 1 and 2Jomar MaisogNo ratings yet
- Gas Processing UnitsDocument5 pagesGas Processing UnitsBashirNo ratings yet
- Steam Generator DesignDocument147 pagesSteam Generator Designzerocool86100% (1)
- 4th Periodical Test Chemistry 1 2017 2018 (Mid Term)Document15 pages4th Periodical Test Chemistry 1 2017 2018 (Mid Term)Marjorie BrondoNo ratings yet
- Cyclones: Cyclone Efficiency Generally Increases WithDocument12 pagesCyclones: Cyclone Efficiency Generally Increases Withnkhanzada100% (1)
- Chemistry - Test 1Document11 pagesChemistry - Test 1SuryaKanta HazraNo ratings yet
- PP Ziauddin BoardDocument28 pagesPP Ziauddin BoardMuhammad ArsalanNo ratings yet
- Barker 2019 Trial PaperDocument63 pagesBarker 2019 Trial PaperYuanfeng WeiNo ratings yet
- 4th Quarter Test Advance ChemDocument4 pages4th Quarter Test Advance ChemJeanisil CerenoNo ratings yet
- HW01 - Group Number - S1 2324 1Document11 pagesHW01 - Group Number - S1 2324 1Như TâmNo ratings yet
- Comprehensive-Chemistry PaperDocument4 pagesComprehensive-Chemistry PaperUmar ZulfiqarNo ratings yet
- HW 03 S1 2324 Group NumberDocument12 pagesHW 03 S1 2324 Group NumberNhư TâmNo ratings yet
- 1st Year Half Book Chem ObjectiveDocument1 page1st Year Half Book Chem ObjectiveRana 001No ratings yet
- Second Quarter Science 9Document7 pagesSecond Quarter Science 9RPONTEJONo ratings yet
- 1st Year CHEMISTRY CH Wise 2021 by 786 AcademyDocument11 pages1st Year CHEMISTRY CH Wise 2021 by 786 AcademySindhu Jatt80% (5)
- Yr. 8 Science Exam Multiple Choice Answer Sheet: Circle The Letter Indicating The Best AnswerDocument16 pagesYr. 8 Science Exam Multiple Choice Answer Sheet: Circle The Letter Indicating The Best AnswerLovy Le ErNo ratings yet
- AIC Form Pro MGTDocument10 pagesAIC Form Pro MGTKiệt ĐỗNo ratings yet
- VBHDocument10 pagesVBHMaricar HababagNo ratings yet
- Chemistry Ssc-I: Answer Sheet No.Document7 pagesChemistry Ssc-I: Answer Sheet No.Mohsin SyedNo ratings yet
- Chem Pu IDocument2 pagesChem Pu IMahesh HugarNo ratings yet
- Maharashtra Board Class 12 Chemistry Question Paper 2023Document4 pagesMaharashtra Board Class 12 Chemistry Question Paper 2023johnhomelander04No ratings yet
- Pretest in General Chemistry 2 MULTIPLE CHOICES: Read and Analyze The Statements and Questions Carefully. Identify The Best OptionDocument2 pagesPretest in General Chemistry 2 MULTIPLE CHOICES: Read and Analyze The Statements and Questions Carefully. Identify The Best OptionSalinas SalinasNo ratings yet
- HW02Document9 pagesHW02Anh Lương QuỳnhNo ratings yet
- Science Test - 1 (Question Paper)Document4 pagesScience Test - 1 (Question Paper)All Bgm MixNo ratings yet
- STD 12 Chemistry 1 Board Question Paper Maharashtra BoardDocument6 pagesSTD 12 Chemistry 1 Board Question Paper Maharashtra BoardTashvi KulkarniNo ratings yet
- Model Paper of Chemistry 9th Class For Peshawar Board PDFDocument2 pagesModel Paper of Chemistry 9th Class For Peshawar Board PDFAfzaal Jan100% (1)
- QP Adv. 2019MT 1 Paper 1 - 16.04.19Document23 pagesQP Adv. 2019MT 1 Paper 1 - 16.04.19menka lisoNo ratings yet
- Dp2223-s1-Es - Chem HL July 2022Document19 pagesDp2223-s1-Es - Chem HL July 2022akilanrameshNo ratings yet
- Chem SSC 2 2nd Half BookDocument4 pagesChem SSC 2 2nd Half BookAsif AyazNo ratings yet
- CHE101 2013-14 Sem1 Test 1Document12 pagesCHE101 2013-14 Sem1 Test 1Botho P. KeosedileNo ratings yet
- CH 2 Test VERSION BDocument6 pagesCH 2 Test VERSION BChazz SatoNo ratings yet
- Chapter 9 QuizDocument6 pagesChapter 9 QuizDuong Nguyen100% (1)
- ChemistryDocument2 pagesChemistryMuhammad AhsanNo ratings yet
- Chem TermDocument3 pagesChem TermAniket SainiNo ratings yet
- 4Q GenChem 1 REVIEWERDocument2 pages4Q GenChem 1 REVIEWERSagi CreatesNo ratings yet
- Exam 2Document23 pagesExam 2Caroline MathewNo ratings yet
- Mike's Videos - General Chemistry Lesson Outline PDFDocument97 pagesMike's Videos - General Chemistry Lesson Outline PDFClarissa BustardeNo ratings yet
- Test-3 (State Board) - (Chem) - Paper - 19.02.2022Document4 pagesTest-3 (State Board) - (Chem) - Paper - 19.02.2022Ammar AnsariNo ratings yet
- Mid - Term 2016 - 2017Document7 pagesMid - Term 2016 - 2017RPONTEJONo ratings yet
- Chemistry HSSC-II - (3rd Set)Document8 pagesChemistry HSSC-II - (3rd Set)Isha KhanNo ratings yet
- HSC 2016 March ChemistryDocument3 pagesHSC 2016 March ChemistryRohit GhereNo ratings yet
- 11 CHEM MCQs 2021 K.BOARDDocument6 pages11 CHEM MCQs 2021 K.BOARDTanveer AhmedNo ratings yet
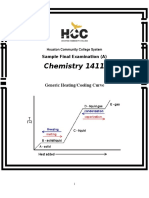
- Chemistry 1411: Generic Heating/Cooling CurveDocument19 pagesChemistry 1411: Generic Heating/Cooling CurveKinal PatelNo ratings yet
- CHM & MTH 101 (Team Anuoluwa)Document29 pagesCHM & MTH 101 (Team Anuoluwa)Oluwabamire Oreoluwa100% (1)
- 12 Model 23Document13 pages12 Model 23wondimuNo ratings yet
- Arada Chemistry 2016 April ModelDocument11 pagesArada Chemistry 2016 April Modelnateesatu4No ratings yet
- CHEMISTRY Model ExamDocument7 pagesCHEMISTRY Model ExamBereket AsefaNo ratings yet
- Problem Set - Day 1 Physical and Chemical PrinciplesDocument16 pagesProblem Set - Day 1 Physical and Chemical PrinciplesLARRY JOHN COMPETENTENo ratings yet
- 2014 H2 Chem Promo (DHS) - PKDocument37 pages2014 H2 Chem Promo (DHS) - PKdragon slayerNo ratings yet
- HCH111 Assignment 2020 Acid BaseDocument7 pagesHCH111 Assignment 2020 Acid BaseBonita NengweNo ratings yet
- Soal Kuis Pengantar Teknik Kimia 2013Document4 pagesSoal Kuis Pengantar Teknik Kimia 2013shawn iceNo ratings yet
- RTS PMR Question Bank Chapter 1 2008-EditedDocument5 pagesRTS PMR Question Bank Chapter 1 2008-EditedmohamedsehatNo ratings yet
- 2 - KHTNDocument10 pages2 - KHTNHải LinhhNo ratings yet
- 717Document13 pages717Himanshu GoelNo ratings yet
- Chemistry G-11 Final ExamDocument4 pagesChemistry G-11 Final ExamGemechu JebesaNo ratings yet
- Chemistry HSSC-II (2nd Set)Document7 pagesChemistry HSSC-II (2nd Set)SAAD RIAZNo ratings yet
- HSC 2016 March ChemistryDocument3 pagesHSC 2016 March ChemistryYSDNo ratings yet
- Journal Skripsi Muhammad Lutfi (F1C112041) PDFDocument10 pagesJournal Skripsi Muhammad Lutfi (F1C112041) PDFBELAJAR BERSAMA. NETNo ratings yet
- MP96 FluidDocument1 pageMP96 Fluidmehmet demirkolNo ratings yet
- Metal Casting SeminarDocument30 pagesMetal Casting SeminarAshish Jadhav0% (1)
- Solution Manual For Chemistry The Science in Context 5th Edition Thomas R Gilbert Rein V Kirss Natalie Foster Stacey Lowery Bretz Geoffrey DaviesDocument77 pagesSolution Manual For Chemistry The Science in Context 5th Edition Thomas R Gilbert Rein V Kirss Natalie Foster Stacey Lowery Bretz Geoffrey DaviesAngelKelleycfayoNo ratings yet
- Lecture 1 Choke PerformanceDocument45 pagesLecture 1 Choke PerformancearvNo ratings yet
- Belajar KaznaeDocument277 pagesBelajar KaznaewahyuNo ratings yet
- RLC GAPAN-2F-LPG PLAN - REVISED ROUTE r1Document1 pageRLC GAPAN-2F-LPG PLAN - REVISED ROUTE r1sharon deramasNo ratings yet
- Heat Transfer. Convection in Pipes. Thermal Conductivity of Celular Insulations. VB FunctionsDocument77 pagesHeat Transfer. Convection in Pipes. Thermal Conductivity of Celular Insulations. VB FunctionsvyrgoNo ratings yet
- Dady SiswayaDocument2 pagesDady SiswayacaknurmNo ratings yet
- Comprehensive Examination in HydraulicsDocument1 pageComprehensive Examination in HydraulicsJordan MagalaNo ratings yet
- Form 4 Chapter 1 Paper 1Document13 pagesForm 4 Chapter 1 Paper 1FakhriahNo ratings yet
- BKF2423 Heat Transfer Sem 2 1718Document5 pagesBKF2423 Heat Transfer Sem 2 1718Tony AngNo ratings yet
- Hydrogen Production With Carbon Dioxide Capture by Reforming of Natural Gas Using Chemical-Looping TechnologiesDocument61 pagesHydrogen Production With Carbon Dioxide Capture by Reforming of Natural Gas Using Chemical-Looping TechnologiesFlorencia CapurroNo ratings yet
- Me8351 Manufacturing Technology-I SyllabusDocument1 pageMe8351 Manufacturing Technology-I SyllabusAnonymous ZB6qyhD6No ratings yet
- ME320 Professor John M. Cimbala: Water Draining From A TankDocument6 pagesME320 Professor John M. Cimbala: Water Draining From A TankShohag HossainNo ratings yet
- Hanbell MPV Valve Spec SheetDocument1 pageHanbell MPV Valve Spec SheetDũng LêNo ratings yet
- Module 1.2 H M T - CONDUCTIONDocument12 pagesModule 1.2 H M T - CONDUCTIONandreslloydralfNo ratings yet
- Daftar Harga E-Katalog Airpurifier HepafilterDocument6 pagesDaftar Harga E-Katalog Airpurifier HepafilterVengky UtamiNo ratings yet
- Daftar Customer PT. ENVICONDocument12 pagesDaftar Customer PT. ENVICONproduksienviconekatamaNo ratings yet
- اساسيات هندسة انتاج النفط والغاز-محول (051-075)Document25 pagesاساسيات هندسة انتاج النفط والغاز-محول (051-075)روان الباشاNo ratings yet
- " Seperation Process": Seminar OnDocument28 pages" Seperation Process": Seminar OnKabilanNo ratings yet
- Iodination of AcetoneDocument5 pagesIodination of Acetonearyajs2017No ratings yet
- Válvulas - Portfólio FT 257-5Document6 pagesVálvulas - Portfólio FT 257-5Compras ServdrillNo ratings yet
- Water InjectionDocument15 pagesWater InjectionKazi EskendirovNo ratings yet
- Bar Chart ProgrammeDocument1 pageBar Chart Programmeakmal sorryNo ratings yet
- Ammonia SYN Catalyst JM Q and ADocument4 pagesAmmonia SYN Catalyst JM Q and AMaged HegabNo ratings yet