Professional Documents
Culture Documents
Disolusi Nazilah
Disolusi Nazilah
Uploaded by
nelvira wulansi0 ratings0% found this document useful (0 votes)
10 views1 pageThe document describes the dissolution of a compound over time. It first calculates the concentration of the compound after 5 minutes of dissolution as 124.2 mg, or 24.84% of the original 500 mg amount. It then calculates the concentration after 10 minutes of dissolution as 362 ug/ml, using the relationship between dissolution concentration and time.
Original Description:
Original Title
Disolusi nazilah
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes the dissolution of a compound over time. It first calculates the concentration of the compound after 5 minutes of dissolution as 124.2 mg, or 24.84% of the original 500 mg amount. It then calculates the concentration after 10 minutes of dissolution as 362 ug/ml, using the relationship between dissolution concentration and time.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageDisolusi Nazilah
Disolusi Nazilah
Uploaded by
nelvira wulansiThe document describes the dissolution of a compound over time. It first calculates the concentration of the compound after 5 minutes of dissolution as 124.2 mg, or 24.84% of the original 500 mg amount. It then calculates the concentration after 10 minutes of dissolution as 362 ug/ml, using the relationship between dissolution concentration and time.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
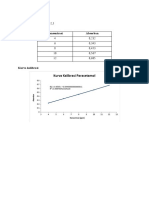
Disolusi
X=6,90 ug/ml × 20 pengenceran
=138 yg/ml
Kadar zat terdisolusi =
KD= X•V+FK
KDt⁵= 138 ug/ml × 900ml
=124.200 ug
=124,2 mg
%terdisolusi = kadar yang di peroleh/kadar sebenarnya × 100%
=124,2 mg/500 mg × 100%
= 24,84%
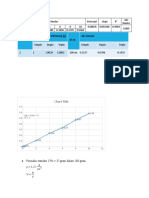
10 menit = 0,44 = 0,0707x - 0,0572
0,5122 = 0,0707
X= 7,24ug/ml
X= 7,24 ug/ml × 50 pengenceran
X= 362ug/ml
KDt10
You might also like
- Total Flavonoid BG DennyDocument13 pagesTotal Flavonoid BG DennyBe GraphNo ratings yet
- Laporan Praktikum Kimia Pangan: "Uji Kadar Protein"Document10 pagesLaporan Praktikum Kimia Pangan: "Uji Kadar Protein"Widya Ratri Hernanda PutriNo ratings yet
- DisolusiDocument4 pagesDisolusinelvira wulansiNo ratings yet
- Hasil Uji Disolusi.Document9 pagesHasil Uji Disolusi.Nola Ayunda PutriNo ratings yet
- Hasil T (Jam) AbsobansiDocument10 pagesHasil T (Jam) AbsobansiAulia DwiNo ratings yet
- Lampiran 1. Perhitungan Dapar Fosfat: G MR X V G XDocument7 pagesLampiran 1. Perhitungan Dapar Fosfat: G MR X V G XNurul HudaNo ratings yet
- Hasil PercobaanDocument7 pagesHasil PercobaanraudahNo ratings yet
- Lampiran Perhitungan Kadar Vit CDocument40 pagesLampiran Perhitungan Kadar Vit Csehat dwi prasajaNo ratings yet
- Rata Rata Kadar 6,2236 × 10 M: 2 G MolDocument2 pagesRata Rata Kadar 6,2236 × 10 M: 2 G MoltheresiaNo ratings yet
- Perhitungan Kadar KotoranDocument8 pagesPerhitungan Kadar KotoranGuita NormiNo ratings yet
- TonisitasDocument6 pagesTonisitasPutri PajarianaNo ratings yet
- Osmolaritas Bobot Awal Zat Bobot Molekul X Jumlahspesies X 100 0 X 1 X 1000 0,30235Document2 pagesOsmolaritas Bobot Awal Zat Bobot Molekul X Jumlahspesies X 100 0 X 1 X 1000 0,30235aris munandarNo ratings yet
- Lampiran Perhitungan Kelompok 1 y 20,10x - 0,172Document16 pagesLampiran Perhitungan Kelompok 1 y 20,10x - 0,172Ridzkia Anggia Putri ElastioNo ratings yet
- Data Pengamatan 1. Perhitungan Larutan IndukDocument4 pagesData Pengamatan 1. Perhitungan Larutan IndukFarah Yumna AmbaroNo ratings yet
- Pulvis (Perhitungan)Document5 pagesPulvis (Perhitungan)IzzaNo ratings yet
- Lembar Kerja 2Document15 pagesLembar Kerja 2Gladis Desyani PutriNo ratings yet
- Data Pengamatan MAI FILLAHDocument5 pagesData Pengamatan MAI FILLAHMuhammad FillahNo ratings yet
- Perhitungan Kurva Baku Teofilin: Chart TitleDocument3 pagesPerhitungan Kurva Baku Teofilin: Chart TitlewahyuNo ratings yet
- A. Penentuan Kadar (Paten)Document6 pagesA. Penentuan Kadar (Paten)roni setiawanNo ratings yet
- Praktikum Biofarmasetika: Data Penetrasi TransdermalDocument6 pagesPraktikum Biofarmasetika: Data Penetrasi TransdermalCindy Riana Putri FebrianiNo ratings yet
- DISOLUSIDocument7 pagesDISOLUSIDinda lestariNo ratings yet
- Masa Soluto VSLN (ML) : Datos: PM Nacl 58.45 G/MolDocument2 pagesMasa Soluto VSLN (ML) : Datos: PM Nacl 58.45 G/Molmoney in the bankNo ratings yet
- Kelompok Bobot Sampel Ditimbang (G) Di Ad Abs SampelDocument4 pagesKelompok Bobot Sampel Ditimbang (G) Di Ad Abs SampelJilan QfNo ratings yet
- Taller #1 Control IDocument10 pagesTaller #1 Control ICLAUDIA TATIANA LONDOÑO CORREANo ratings yet
- Assignment To Analytical TechniquesDocument4 pagesAssignment To Analytical TechniquesBona UwiragiyeNo ratings yet
- Hitung Segitiga WarnaDocument7 pagesHitung Segitiga Warnabakhti akbarNo ratings yet
- Lamp 3 PerhitunganDocument4 pagesLamp 3 PerhitunganRani MukherjiNo ratings yet
- Data Praktikum Spektro MultivariatDocument4 pagesData Praktikum Spektro MultivariatWassis UtamiNo ratings yet
- %rendemen Berat Ekstrak (G) Berat Simplisia (G) ×100 %Document6 pages%rendemen Berat Ekstrak (G) Berat Simplisia (G) ×100 %safiaNo ratings yet
- Bab Iv Hasil Dan Pembahasan 4.1 HASIL 4.1.1 Perhitungan Konsentrasi Larutan BakuDocument8 pagesBab Iv Hasil Dan Pembahasan 4.1 HASIL 4.1.1 Perhitungan Konsentrasi Larutan BakuHafid BiladNo ratings yet
- Penentuan Profil Laju Disolusi Tablet Lepas Lambat AsetosalDocument12 pagesPenentuan Profil Laju Disolusi Tablet Lepas Lambat AsetosalNurlaila SukmawatiNo ratings yet
- Co Berat Glukosa Monohidrat, MG Volume Larutan, ML × BM Glukosa BM Glukosamonohidrat MG MLDocument5 pagesCo Berat Glukosa Monohidrat, MG Volume Larutan, ML × BM Glukosa BM Glukosamonohidrat MG MLYosep ChristianNo ratings yet
- Lampiran Perhitungan KLP 8Document3 pagesLampiran Perhitungan KLP 8Nadia UmmahNo ratings yet
- Lap AnsedDocument5 pagesLap AnsedRuliana AnuNo ratings yet
- Ulangan Berat Smpel (MG) Pengenceran Absorbansi Kadar Cuso (Mg/Tablet)Document3 pagesUlangan Berat Smpel (MG) Pengenceran Absorbansi Kadar Cuso (Mg/Tablet)Zidny IlmayaqinNo ratings yet
- Kurva Kalibrasi Panadol 500 MGDocument5 pagesKurva Kalibrasi Panadol 500 MGMayang Utari D3-2019No ratings yet
- Tugas 4 Statistika FFDocument2 pagesTugas 4 Statistika FFNailin Naja50% (2)
- Tugas QC Dan ValidasiDocument4 pagesTugas QC Dan ValidasiJuw EllahNo ratings yet
- Soal CompoundingDocument16 pagesSoal CompoundingRezaFArthaNo ratings yet
- I. Formula: 1 Hari: 3 - 4 X Sehari 400 MGDocument2 pagesI. Formula: 1 Hari: 3 - 4 X Sehari 400 MGPramuktiNo ratings yet
- Modul P.farmasetik IIDocument9 pagesModul P.farmasetik IIshaniNo ratings yet
- Siti Nafsiatul M - TP3 ASODocument3 pagesSiti Nafsiatul M - TP3 ASOSiti NafsiatulNo ratings yet
- Hasil Pengamatan GulaDocument1 pageHasil Pengamatan GulaNisa FitriNo ratings yet
- Bab Iv Formulasi Dan Perhitungan 4.1 Formulasi: Iso IsoDocument4 pagesBab Iv Formulasi Dan Perhitungan 4.1 Formulasi: Iso IsoIstiqomah Sa'adahNo ratings yet
- Perhitungan DMDocument3 pagesPerhitungan DMAGATA M.H KINANTINo ratings yet
- Calcule Las Concentraciones en MGDocument2 pagesCalcule Las Concentraciones en MGguianella AlarconNo ratings yet
- Uji DisolusiDocument9 pagesUji DisolusiAdhoNo ratings yet
- Laporan Praktikum Farmasi Fisika Kecepatan DisolusiDocument14 pagesLaporan Praktikum Farmasi Fisika Kecepatan DisolusiMaulidha Yuniza AnantaNo ratings yet
- TUGAS 1 - Konversi Satuan PDFDocument14 pagesTUGAS 1 - Konversi Satuan PDFSherly MarliantiNo ratings yet
- Data PengamatanDocument6 pagesData Pengamatanwortelkentang111No ratings yet
- Konsentrasi Absorbansi 3 6 9 12 15 Intercept Slopee: Sxo Vxo % RecoveryDocument3 pagesKonsentrasi Absorbansi 3 6 9 12 15 Intercept Slopee: Sxo Vxo % RecoveryJilan QfNo ratings yet
- Agromulyo 30,59 35,59 35,21 8,2 0,7 0,03 3,92 130Document4 pagesAgromulyo 30,59 35,59 35,21 8,2 0,7 0,03 3,92 130dimasNo ratings yet
- Nama: Shafira Rizqika Ramadhina NPM: 10060317020 Farmasi A Teknologi Sediaan SolidaDocument8 pagesNama: Shafira Rizqika Ramadhina NPM: 10060317020 Farmasi A Teknologi Sediaan Solidabaihaqi shafariNo ratings yet
- Analytical Lab Exp.1Document5 pagesAnalytical Lab Exp.1Mayson BaliNo ratings yet
- Resep KapsulDocument6 pagesResep KapsulMas AwangNo ratings yet
- Disusi 10Document8 pagesDisusi 10Zaqina Erin Setya FazriNo ratings yet
- 2ml 1ml: Volume Akhir VolumeawalDocument8 pages2ml 1ml: Volume Akhir VolumeawalUswatunnisaNo ratings yet
- Bab Iv.1 HasilDocument6 pagesBab Iv.1 HasilCitra KencanaNo ratings yet
- Calculos Pureza Del MagnesioDocument2 pagesCalculos Pureza Del Magnesiokevin 00No ratings yet
- Laporan Praktikum Kimia Fisika I Kenaikan Titik Didih LarutanDocument12 pagesLaporan Praktikum Kimia Fisika I Kenaikan Titik Didih LarutanxaxuxuNo ratings yet