Professional Documents
Culture Documents
Exercise 1 CHM476
Uploaded by
PUTRI DAYANA BATRIESYA ABDUL HANIF0 ratings0% found this document useful (0 votes)
6 views1 pageThe document describes a chemical reaction between carbon monoxide and nitrogen dioxide gases. The forward reaction has an activation energy of 100 kJ/mole and an enthalpy change of -226 kJ/mol. The activation energy for the reverse reaction would also be 100 kJ/mole.
Original Description:
EXERCISE CHM476 PHYSICAL CHEMISTRY MDM SHAFINAS
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes a chemical reaction between carbon monoxide and nitrogen dioxide gases. The forward reaction has an activation energy of 100 kJ/mole and an enthalpy change of -226 kJ/mol. The activation energy for the reverse reaction would also be 100 kJ/mole.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 pageExercise 1 CHM476
Uploaded by
PUTRI DAYANA BATRIESYA ABDUL HANIFThe document describes a chemical reaction between carbon monoxide and nitrogen dioxide gases. The forward reaction has an activation energy of 100 kJ/mole and an enthalpy change of -226 kJ/mol. The activation energy for the reverse reaction would also be 100 kJ/mole.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
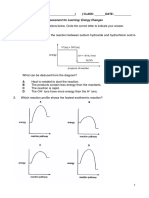
Exercise 1
Sketch a potential-energy diagram to show the reaction profile of the chemical
reaction. The activation energy = 100 kJ/mole.
CO(g) + NO2(g) CO2(g) + NO(g) ∆H = -226 kJ mol-1
a) Indicate the activation energy as well as the enthalpy change of the reaction
b) What is the activation energy for the reverse reaction?
You might also like
- IB Chemistry SL Topic 5 Questions 1Document11 pagesIB Chemistry SL Topic 5 Questions 1Vibha RaviNo ratings yet
- 2020 Chemical Energetics Part 1 TutorialDocument13 pages2020 Chemical Energetics Part 1 TutorialSalman ShethNo ratings yet
- ThermodynamicsDocument28 pagesThermodynamicsJack Lupino100% (2)
- Chemistry Lab# 4 (Completed)Document4 pagesChemistry Lab# 4 (Completed)tahjsalmon100% (1)
- Topic 7-17 Practice Questions Key 1 2Document8 pagesTopic 7-17 Practice Questions Key 1 2Isaline GurneNo ratings yet
- Energetics MCQDocument6 pagesEnergetics MCQCenturo VinNo ratings yet
- Energetics 1 Practice Problems (2024) SOLUTIONSDocument4 pagesEnergetics 1 Practice Problems (2024) SOLUTIONSHakkyu KimNo ratings yet
- 2 Quizizz 2019 ptVIIIe DocDocument10 pages2 Quizizz 2019 ptVIIIe DocKM Tsang Ka ManNo ratings yet
- Energetics SL - MCQDocument22 pagesEnergetics SL - MCQAster LeeNo ratings yet
- Unit 5 MCQSDocument27 pagesUnit 5 MCQSFiras Ahmad100% (2)
- HW - Energy Diagram Worksheet: 1. Using The Energy Curve Below The Label and Answer The Following QuestionsDocument3 pagesHW - Energy Diagram Worksheet: 1. Using The Energy Curve Below The Label and Answer The Following QuestionsAlph BrickNo ratings yet
- Chem Energetics TestDocument7 pagesChem Energetics TestJkaurbhsNo ratings yet
- Example Problems For Lecture 15: Umass Boston Chem 115 Spring 2009 Prof. SevianDocument4 pagesExample Problems For Lecture 15: Umass Boston Chem 115 Spring 2009 Prof. Seviankamal touilebNo ratings yet
- 2.0 Thermochemistry Dec 21Document77 pages2.0 Thermochemistry Dec 21Shaarmini SankerNo ratings yet
- (Total 1 Mark) : H - 390 KJ H - 520 KJDocument11 pages(Total 1 Mark) : H - 390 KJ H - 520 KJRonald McdonaldNo ratings yet
- Chemical Energetics (As) 2023 With AnswersDocument12 pagesChemical Energetics (As) 2023 With AnswersNurli SallehNo ratings yet
- Chemical Reactions and HeatDocument37 pagesChemical Reactions and HeatDamir BalmassovNo ratings yet
- 2223 Grade 10 Chemistry Chapter 8 NotesDocument12 pages2223 Grade 10 Chemistry Chapter 8 NotesZa Evolution ClanNo ratings yet
- Lecture 1Document56 pagesLecture 1Izzad Zuhair IsmailNo ratings yet
- Energetics QuestionsDocument58 pagesEnergetics QuestionsQasim Peracha100% (1)
- Chapter 34Document66 pagesChapter 34Nina FairuzNo ratings yet
- Energy & Rates Worksheet 5Document1 pageEnergy & Rates Worksheet 5feras.barbar06No ratings yet
- G = Δh - Tδs: Spontaneity and Gibbs free energyDocument4 pagesG = Δh - Tδs: Spontaneity and Gibbs free energyAdufe RufaiNo ratings yet
- Year 12 Enthalpy Changes February Half TermDocument28 pagesYear 12 Enthalpy Changes February Half TermEri-ife OlufemiNo ratings yet
- Tutorial Module Sk025: Chemistry Semester 2 Chapter 2.0: Thermochemistry Unit 2.1: Concept of EnthalpyDocument7 pagesTutorial Module Sk025: Chemistry Semester 2 Chapter 2.0: Thermochemistry Unit 2.1: Concept of EnthalpyMUHAMMAD IMRONNo ratings yet
- Energetics Q + MSDocument32 pagesEnergetics Q + MSmamta2111No ratings yet
- Chapter 5.1 OHDocument5 pagesChapter 5.1 OHMichelle NgNo ratings yet
- 3.2.1 Enthalpy Changes QP MultiDocument21 pages3.2.1 Enthalpy Changes QP MultiHadiyaNo ratings yet
- T5 (54 Marks) : 1. (1 Mark)Document18 pagesT5 (54 Marks) : 1. (1 Mark)Ege DumluNo ratings yet
- Reaction Mechanisms Catalysts Worksheet Solutions 12ph5x4Document3 pagesReaction Mechanisms Catalysts Worksheet Solutions 12ph5x4nicole100% (1)
- Lecture 12. Reactive SystemsDocument9 pagesLecture 12. Reactive SystemsHirun ManujayaNo ratings yet
- Chapter 15 and 16 Revision: (104 Marks)Document26 pagesChapter 15 and 16 Revision: (104 Marks)aurennosNo ratings yet
- Enthalpy Changes 2Document1 pageEnthalpy Changes 218bhattzNo ratings yet
- Energetics I (Multiple Choice) QPDocument16 pagesEnergetics I (Multiple Choice) QPsarahNo ratings yet
- Final Revision WorksheetDocument26 pagesFinal Revision Worksheetawash0takuNo ratings yet
- Asc 2016Document25 pagesAsc 2016Samruddhi MohiteNo ratings yet
- Thermodynamics: I Puc - Chemistry Chapter - 06Document11 pagesThermodynamics: I Puc - Chemistry Chapter - 06Udaybhaskar LalamNo ratings yet
- PDF PDF nergeticsReviewQuestions (2024)Document10 pagesPDF PDF nergeticsReviewQuestions (2024)Hakkyu KimNo ratings yet
- Chapter 9 - Termochemistry 55Document55 pagesChapter 9 - Termochemistry 55ABC_Ais Batu CampurNo ratings yet
- EnergeticsDocument35 pagesEnergeticsRodaine BaileyNo ratings yet
- Kesetimbangan KimiaDocument19 pagesKesetimbangan KimiaAmel RahmaNo ratings yet
- Long Answer Type QNSDocument2 pagesLong Answer Type QNSkavisanjurohillaNo ratings yet
- KD 1: Hydrocarbon and Crude OilDocument4 pagesKD 1: Hydrocarbon and Crude OilJocellyn AurelliaNo ratings yet
- Ch5.3 (16 Marks) : 1. (1 Mark)Document6 pagesCh5.3 (16 Marks) : 1. (1 Mark)The LegendaryCarrotMasterNo ratings yet
- Chemistry Sk025 SESSION 2019/2020 Topic: ThermochemistryDocument3 pagesChemistry Sk025 SESSION 2019/2020 Topic: ThermochemistryHaiyi GohNo ratings yet
- EnergeticsDocument6 pagesEnergeticsAmmar AbiddNo ratings yet
- Chemistry 7Document17 pagesChemistry 7Jong.Gun.KimNo ratings yet
- 1 - Kinetic and Potential EnergyDocument3 pages1 - Kinetic and Potential Energydenzelf2No ratings yet
- Enthalpy Review QuestionsDocument3 pagesEnthalpy Review Questionsranjana roy100% (1)
- Hsslive-Xi-Chem-Prvs-Qn-6. Thermodynamics Q & ADocument10 pagesHsslive-Xi-Chem-Prvs-Qn-6. Thermodynamics Q & AshineNo ratings yet
- 10 Thermodynamics (S)Document8 pages10 Thermodynamics (S)Mr TanNo ratings yet
- 15.2 (A) Entropy and SpontaneityDocument6 pages15.2 (A) Entropy and SpontaneityKshiraj PanchalNo ratings yet
- Answer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerDocument4 pagesAnswer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerTimothy HandokoNo ratings yet
- Review Questions EnergeticsDocument13 pagesReview Questions EnergeticsSoobinNo ratings yet
- Hsslive-Xi-Chem-5. Thermodynamics Q & ADocument11 pagesHsslive-Xi-Chem-5. Thermodynamics Q & AarnadicgamingNo ratings yet
- Thermochemistry PC EDocument12 pagesThermochemistry PC Eb72hbapqiNo ratings yet
- Chapter 5 and 6 Questions: (58 Marks)Document21 pagesChapter 5 and 6 Questions: (58 Marks)aurennosNo ratings yet
- Unit 6 MCQsDocument22 pagesUnit 6 MCQsFiras AhmadNo ratings yet
- 7 Chemical EnergeticsDocument176 pages7 Chemical EnergeticsUng Hie HuongNo ratings yet
- Intro To Organic Reactions Chm457Document52 pagesIntro To Organic Reactions Chm457PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chapter 2 CHM476 (Part 2)Document15 pagesChapter 2 CHM476 (Part 2)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chapter 2 CHM476 (Part 1)Document16 pagesChapter 2 CHM476 (Part 1)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chapter 1 CHM476 (Part 2)Document13 pagesChapter 1 CHM476 (Part 2)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chapter 1 CHM476 (Part 3 and 4)Document25 pagesChapter 1 CHM476 (Part 3 and 4)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Chapter 1 CHM476 (Part 1)Document28 pagesChapter 1 CHM476 (Part 1)PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Intro To Organic Reactions CHM457Document52 pagesIntro To Organic Reactions CHM457PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Tutorial 1 CHM476Document3 pagesTutorial 1 CHM476PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Topic 3 - CHM421Document68 pagesTopic 3 - CHM421PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet
- Topic 2 Cleaning Solutions CHM421Document6 pagesTopic 2 Cleaning Solutions CHM421PUTRI DAYANA BATRIESYA ABDUL HANIFNo ratings yet