Professional Documents
Culture Documents
CHEM 02 2nd Year Exam
Uploaded by
mt khanOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHEM 02 2nd Year Exam
Uploaded by
mt khanCopyright:
Available Formats
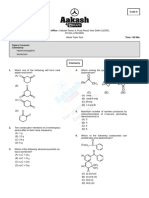
AXIS PUBLIC HIGH SCHOOL FAROOQABAD
EASTERN RAILWAY CROSSING BYPASS FAROOQABAD, SHEIKHUPURA
Student Name Roll Num Class Name Paper Code
INTER-II
Subject Name Time Allowed Total Marks Exam Date
Chemistry 34 18-Apr-2023
Exam Syllabus
CHAP 9
1- 2- 3- 4-
5- 6- 7- 8-
9- 10 -
Q1. Choose the correct answer. 1X10=10
1. Caol tar is the main source of: (A) (B) (C) (D)
Aromatic Aliphatic Non-Aromatic None of these
compounds compounds compounds
2. The m-xylene is also called: (A) (B) (C) (D)
1,2 dimethyl 1,4-dimethyl 1,3- dimethyl 1,6-dimethy
benzene benzene benzene benzene
3. Benzene was discovered by: (A) Hoffman (B) (C) Kekule (D)
Micheal None of them
faraday
4. How many sigma and pi bonds are present in benzene molecule? (A) (B) (C) (D)
6 sigma 2 pi 9 sigma 3 pi 12 sigma 6 pi 12 sigma 3 pi
5. The thre alternate double and sigle bonds in benzene stucture (A) (B) (C) (D)
are called: Conjugate Resonating Both a and b None of these
bonds bonds
6. In benzene ring the number of sigma bond , which is formed by (A) 4 (B) 6 (C) 8 (D) 10
2
sp -s overlapping are?
7. The extra stability of benzene is due to: (A) (B) (C) (D)
Presence of Delocalization Ring Three double
sigma bond of pi electrons structures bond
8. Amongst the following polycyclic compound is: (A) Xylene (B) (C) Styrene (D) Cumene
Naphthalene
9. Acylation of benzene to product aliphatic aromatic ketones is (A) (B) (C) (D)
known as: Insoluble in Soluble in Electron Electron rich
chloride and ether deficient
aluminium ion
10. Among the following the compound that can be sulphonated (A) Toluene (B) Benzene (C) (D)
most easily is: Nitro benzene Chloro
benzene
Q2. Write short answers of the following questions. (Any 8) 2X8=16
I . Define aromatic hydrocarbons? II . Write any four examples of polycyclic aromatic but not
monocyclic aromatic hydrocarbons why.
III . Aromatic compounds burn with sooty flame.justify. IV . Write down resonating structures of benzene.
V . What is aromatization? VI . Define resonance energy. what is the resonance of benzene?
VII . Convert cyclohexane → benzene. VIII . How prepare toluene from n-hexane.
IX . How prepared benzene from phenol. X . Define sulphonation with examples?
Q3. Write detailed answers of the following questions. 4X2=8
1. Draw the structural formula for the following compounds. (i) m- 2. Detail out three reactions in which benzene behaves as if it is a
chlorobenzene (ii) p-hydroxybenzonic (iii) o- saturated hydrocarbons and three reactions in which it behaves
Bromonitrobenzene (iv) o- Ethyltotuene (v) p-Nitroaniline (vi) as if it is unsaturated.
2,4,5- Trinitrotoluene (vii) m-Nitrphenol (viii) p-dibenzylbenzene
(ix) 2-Amino-5-bromo-3 nitrobenzesulphonic acid. (b) Give names
and the possible isomeric structures of the following. (i) Xylenes
(ii) Trimethylbenzne (iii) Bromonitrotoluene
You might also like
- 2nd Year ChemistryDocument2 pages2nd Year ChemistryTariq RayNo ratings yet
- The Principles of Heterocyclic ChemistryFrom EverandThe Principles of Heterocyclic ChemistryRating: 3 out of 5 stars3/5 (2)
- Carbon CompoundsDocument3 pagesCarbon CompoundsShreya AjithNo ratings yet
- ChemDocument39 pagesChemJaimin Senta100% (1)
- 6417 Topper 21 129 510 2 8532 Isomerism Up201612091817 1481287659 483 PDFDocument41 pages6417 Topper 21 129 510 2 8532 Isomerism Up201612091817 1481287659 483 PDFMd Waquar SalisNo ratings yet
- 13 DPP 01a Mixed Boc ExcelDocument5 pages13 DPP 01a Mixed Boc ExcelKiller ẞunnyNo ratings yet
- CH102 Principles and Reactions in Organic Chemistry: Fste School of Biological and Chemical SciencesDocument13 pagesCH102 Principles and Reactions in Organic Chemistry: Fste School of Biological and Chemical SciencesTetzNo ratings yet
- 1001 CHM 102 CBT Practice QuestionDocument84 pages1001 CHM 102 CBT Practice QuestionBig Peace???No ratings yet
- 9553373d-9a91-436b-9882-56fb21f3a4dbDocument2 pages9553373d-9a91-436b-9882-56fb21f3a4dbSagar AhireNo ratings yet
- Chemistry - Mains2 (Entire 11th)Document7 pagesChemistry - Mains2 (Entire 11th)Ravi Kiran KoduriNo ratings yet
- Overall CHP 7 and 12Document4 pagesOverall CHP 7 and 12faheemNo ratings yet
- Chemistry 9th Class F.B (TCI)Document4 pagesChemistry 9th Class F.B (TCI)Mohammad AshfaqNo ratings yet
- Neet 18-1Document5 pagesNeet 18-1h47xa4t5No ratings yet
- CBSE Class 10 Term 2 Carbon and Its Compounds Subjective QuestionsDocument2 pagesCBSE Class 10 Term 2 Carbon and Its Compounds Subjective QuestionsPahal Kumari SinhaNo ratings yet
- Jee Main 28with Answer 29 26 Feb 2C 2021 Shift 2Document43 pagesJee Main 28with Answer 29 26 Feb 2C 2021 Shift 2CREATIVE XNo ratings yet
- T9 2nd Year Chapter Wise Test Chemistry Chapter 9 2nd YearDocument1 pageT9 2nd Year Chapter Wise Test Chemistry Chapter 9 2nd Yearzubairthair249No ratings yet
- MCQ Chemistry Practice Qwestions Class 12thDocument8 pagesMCQ Chemistry Practice Qwestions Class 12thMithun ChakladarNo ratings yet
- Organic Chemistry MCQSDocument14 pagesOrganic Chemistry MCQSbsat-f21-242No ratings yet
- Latihan SkoDocument17 pagesLatihan Skorusnah chungNo ratings yet
- 116180HSSC IichemistryDocument2 pages116180HSSC IichemistryMughal usmanNo ratings yet
- Classifi & Nome Exercise Module-3-2Document21 pagesClassifi & Nome Exercise Module-3-2Raju SinghNo ratings yet
- ISC 5 Years Chemistry-1Document8 pagesISC 5 Years Chemistry-1man3658anNo ratings yet
- A Level Chemistry Paper 2 Exam 17Document4 pagesA Level Chemistry Paper 2 Exam 17Anthony AndyNo ratings yet
- ChemistryDocument7 pagesChemistryrjakrithiNo ratings yet
- JEE Main Full Mock Test 8Document10 pagesJEE Main Full Mock Test 8Aditya SinghNo ratings yet
- HSC Chemistry 2014 Part 2Document2 pagesHSC Chemistry 2014 Part 2Tashvi KulkarniNo ratings yet
- CSN X Carbon ND and VagDocument2 pagesCSN X Carbon ND and VagDeena chemistNo ratings yet
- Ex 3Document4 pagesEx 3VIKRANTH KUMAR JAKKOJUNo ratings yet
- STD 12 Chemistry 2 Board Question Paper Maharashtra BoardDocument10 pagesSTD 12 Chemistry 2 Board Question Paper Maharashtra BoardYash ChaudhauryNo ratings yet
- Cbjescco 04Document8 pagesCbjescco 04Soni MehtaNo ratings yet
- Chem 1001Document2 pagesChem 1001sudarshan kumar chaudharyNo ratings yet
- SQP 20 Sets ChemistryDocument144 pagesSQP 20 Sets Chemistrypoornima9739100% (1)
- HSSC 2 FEDERALDocument4 pagesHSSC 2 FEDERALShahid Ur RehmanNo ratings yet
- IsomerismDocument4 pagesIsomerismNaziya KosarNo ratings yet
- Class 12 ChemistryDocument16 pagesClass 12 ChemistrysipherbizNo ratings yet
- Sample Paper Chem3333333333333333333Document1 pageSample Paper Chem3333333333333333333maria b chackoNo ratings yet
- SQP 20 Sets ChemistryDocument145 pagesSQP 20 Sets ChemistrySky Sir50% (4)
- 2023-2024 Pre BoardDocument14 pages2023-2024 Pre BoardKalai SelviNo ratings yet
- Chapter 16 (Wade)Document2 pagesChapter 16 (Wade)Mau BaraquelNo ratings yet
- @bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDDocument5 pages@bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDxkryxxzNo ratings yet
- STD 12 Chemistry 2 Board Question Paper Maharashtra BoardDocument10 pagesSTD 12 Chemistry 2 Board Question Paper Maharashtra BoardTashvi KulkarniNo ratings yet
- Cblechpu 02Document11 pagesCblechpu 02Free FireNo ratings yet
- Adobe Scan 23 Dec 2022Document7 pagesAdobe Scan 23 Dec 2022GAURAV kumarNo ratings yet
- Bio MoleculesDocument2 pagesBio MoleculesAnonymous OulpeHpWVrNo ratings yet
- 11DPP01FCOMBINEDEMERGEDocument3 pages11DPP01FCOMBINEDEMERGEAyush KumarNo ratings yet
- Chemistry XII Pre-Board 1 (23-24)Document10 pagesChemistry XII Pre-Board 1 (23-24)leothiveshNo ratings yet
- Chem ch4Document17 pagesChem ch4Winter GamingNo ratings yet
- ChemistryDocument10 pagesChemistryprasanth kNo ratings yet
- Test Paper On Chapter 9 Coordination Compounds Class 12 ChemistryDocument2 pagesTest Paper On Chapter 9 Coordination Compounds Class 12 ChemistryjacksucksatlifeNo ratings yet
- DPT 33 Centre Rasi Iit Jee Che Key 09-12-23Document4 pagesDPT 33 Centre Rasi Iit Jee Che Key 09-12-23Deena chemistNo ratings yet
- CB - 8Document4 pagesCB - 8gginrearrangeitproperlyNo ratings yet
- Number O-Bonds Present: (A) SP, SP?Document6 pagesNumber O-Bonds Present: (A) SP, SP?friendship friendshipNo ratings yet
- Allen: Code: A-3 Kcet - 2020 Test Paper With Answer Key (Held On Friday 31july, 2020)Document6 pagesAllen: Code: A-3 Kcet - 2020 Test Paper With Answer Key (Held On Friday 31july, 2020)Raja Simha JNNo ratings yet
- Chem Wa2Document2 pagesChem Wa2Balarama RajuNo ratings yet
- Adobe Scan Feb 28, 2023Document11 pagesAdobe Scan Feb 28, 2023Vikram NeelmegamNo ratings yet
- Foundations of Organic Chemistry (Transcript) by Ron B. Davis Jr.Document2 pagesFoundations of Organic Chemistry (Transcript) by Ron B. Davis Jr.Sravan SmartNo ratings yet
- Atomic Energy Central School-Kudankulam: Time Allowed: 1 Hour and 30 Minutes Maximum Marks: 35 General InstructionsDocument7 pagesAtomic Energy Central School-Kudankulam: Time Allowed: 1 Hour and 30 Minutes Maximum Marks: 35 General Instructions39 Yogendra KumarNo ratings yet
- (4104) DPP 32 50 BDocument20 pages(4104) DPP 32 50 BRAJDEEP DASNo ratings yet
- 2nd Year ExamsDocument2 pages2nd Year Examsmt khanNo ratings yet
- 2nd Year Exams EngDocument1 page2nd Year Exams Engmt khanNo ratings yet
- 6-Cuprammonium RayonDocument21 pages6-Cuprammonium Rayonmt khanNo ratings yet
- Textile 5-Viscose RayonDocument21 pagesTextile 5-Viscose Rayonmt khanNo ratings yet
- TT 403 Ginning TechnologyDocument45 pagesTT 403 Ginning Technologymt khan100% (2)
- Jute As A Vegetable Textile Fibre1Document74 pagesJute As A Vegetable Textile Fibre1mt khanNo ratings yet
- Textile 302 TheoryDocument43 pagesTextile 302 Theorymt khanNo ratings yet
- Earn Money Online 100 Percent Gurrenty Payment and Work Totally Free. Just Take A Chance Its Real. Use Link To RegisterDocument1 pageEarn Money Online 100 Percent Gurrenty Payment and Work Totally Free. Just Take A Chance Its Real. Use Link To Registermt khanNo ratings yet
- Pesticide Synthesis Handbook - Tebuconazole (1996)Document1 pagePesticide Synthesis Handbook - Tebuconazole (1996)Led TassoNo ratings yet
- How To Layer The Ordinary 7Document2 pagesHow To Layer The Ordinary 7Neha RNo ratings yet
- 8 - Enxofre Na AgriculturaDocument12 pages8 - Enxofre Na AgriculturaEudson VictorNo ratings yet
- Soil Sample Analysis Methods Shaon Kumar DasDocument19 pagesSoil Sample Analysis Methods Shaon Kumar DasPoonam JaiswalNo ratings yet
- Biochemistry 9th Edition by Campbell Farrel and McDougal ISBN Test BankDocument25 pagesBiochemistry 9th Edition by Campbell Farrel and McDougal ISBN Test Bankdoris100% (23)
- TranslationDocument51 pagesTranslationAleena MustafaNo ratings yet
- Chemistry 1 11 Q2 M13Document14 pagesChemistry 1 11 Q2 M13Jessie CandawanNo ratings yet
- CatalogueDocument3 pagesCatalogueChristian AlmengorNo ratings yet
- 10 1016@j Tifs 2019 12 004 PDFDocument38 pages10 1016@j Tifs 2019 12 004 PDFJuan E SotoNo ratings yet
- CBSE Class 10 Chemistry Worksheet - Carbon and Its CompoundDocument4 pagesCBSE Class 10 Chemistry Worksheet - Carbon and Its Compoundomm2500100% (1)
- NOTES 3 The Stages of Cellular RespirationDocument20 pagesNOTES 3 The Stages of Cellular RespirationJillian Reyes SantosNo ratings yet
- Introducing Our New Innovative Cosmetic Loose Powder To Beautify Eyes and Control AcneDocument26 pagesIntroducing Our New Innovative Cosmetic Loose Powder To Beautify Eyes and Control AcneAsep Syaefun NazmiNo ratings yet
- Functional Group InterconversionDocument4 pagesFunctional Group InterconversionPG ChemistryNo ratings yet
- 6C. AntioxidantsDocument2 pages6C. AntioxidantsKim Xiarisse BalugayNo ratings yet
- Scientific African: Joachim M. Dotto, James S. ChachaDocument14 pagesScientific African: Joachim M. Dotto, James S. Chacharetno kusmaNo ratings yet
- Topic 1 SlopDocument80 pagesTopic 1 SlopMaggie PampinNo ratings yet
- Erp 4 Ef 565 F 50197 DCDocument92 pagesErp 4 Ef 565 F 50197 DCsingkorn samansukNo ratings yet
- Aqa Chem4 QP Jan13 PDFDocument24 pagesAqa Chem4 QP Jan13 PDFMazlinNo ratings yet
- C33LT D10D03029 012010Document2 pagesC33LT D10D03029 012010metaslaNo ratings yet
- Live All Questions Final2021Document50 pagesLive All Questions Final2021Hab AnneNo ratings yet
- Aerobic Metabolism of Lactic Acid BacteriaDocument12 pagesAerobic Metabolism of Lactic Acid Bacteriaadel strikeNo ratings yet
- Acetone MSDSDocument11 pagesAcetone MSDSBrian GardnerNo ratings yet
- 4 Sfa Chemistry N Matsci MRKDocument826 pages4 Sfa Chemistry N Matsci MRKFatimaezzahra FaouziNo ratings yet
- Amoran PCTS-5 Act-2 PDFDocument7 pagesAmoran PCTS-5 Act-2 PDFAnnie Jane P. DosanoNo ratings yet
- Total Synthesis of NorzoanthamineDocument7 pagesTotal Synthesis of NorzoanthamineAyush BoseNo ratings yet
- Biodiesel Production PHD ThesisDocument6 pagesBiodiesel Production PHD Thesisafcngxbbx100% (2)
- Determination of Propionates and Propionic Acid in Bakery Products Using Gas ChromatographyDocument7 pagesDetermination of Propionates and Propionic Acid in Bakery Products Using Gas ChromatographyNurul Mukhlisa AmirNo ratings yet
- LL SolvotrodeDocument3 pagesLL SolvotrodeahmedNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced Level in Chemistry (9701)Document18 pagesCambridge International Advanced Subsidiary and Advanced Level in Chemistry (9701)GregNo ratings yet
- Metabolism of Lipids 2Document71 pagesMetabolism of Lipids 2Mi PatelNo ratings yet
- ICH Quality Guidelines: An Implementation GuideFrom EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (1)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- Guidelines for Defining Process Safety Competency RequirementsFrom EverandGuidelines for Defining Process Safety Competency RequirementsRating: 3 out of 5 stars3/5 (1)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesFrom EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesNo ratings yet
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesFrom EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesRating: 5 out of 5 stars5/5 (2)