Professional Documents
Culture Documents
Activity 1
Uploaded by
Charlie Puth0 ratings0% found this document useful (0 votes)
11 views1 pagehfdf
Original Title
ACTIVITY 1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenthfdf
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views1 pageActivity 1
Uploaded by
Charlie Puthhfdf
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
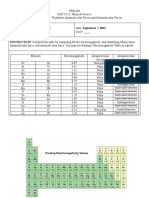
ACTIVITY 1: BOND POLARITY AND INTERMOLECULAR FORCES OF ATTRACTION Objectives At the end of this activity, students
are expected to be able to: Compare and contrast polar and nonpolar molecules; Investigate the difference between
Intramolecular forces and Intermolecular forces of attraction; and Investigate the different types of Intermolecular Forces
and how they relate to their physical properties. PART 1. Complete the following table by indicating the electronegativity
difference of the atom present, drawing the dipole moment by indicating the partial positive side and partial negative side,
and identifying the type of bond present on the compound
Molecule Electronegativity Difference Dipole Moment Type of Bond
CO2 0.89 0 two covalent bonds
HF 4.0 1.8D POLAR COVALENT BOND
H20 1.5 1.84D COVALENT BONDS
CH4 0 covalent bonds
NaF 3 0.24 D ionic bond
You might also like
- 3 PolarityDocument18 pages3 PolarityCarlo PrigoNo ratings yet
- Bonds IIIDocument2 pagesBonds IIImanoranjan838241No ratings yet
- Chemical Bonding-Part 2Document12 pagesChemical Bonding-Part 2Gertrude Yasha Anne RosalesNo ratings yet
- Intermolecular Forces:: Attraction in Molecular LevelDocument34 pagesIntermolecular Forces:: Attraction in Molecular LevelKelly MarceloNo ratings yet
- Module 5 Polarity and Itermolecular ForcesDocument28 pagesModule 5 Polarity and Itermolecular ForcesJhon AlbadosNo ratings yet
- Chapter 3 Chemical Bonding and StructureDocument11 pagesChapter 3 Chemical Bonding and Structurenesrine boufadenNo ratings yet
- Predict Polarity With Electronegativity DiffDocument2 pagesPredict Polarity With Electronegativity DiffRaymond LiuNo ratings yet
- M1a1 EstradaDocument1 pageM1a1 EstradaAsia EstradaNo ratings yet
- 5.types of Covalent CompoundsDocument17 pages5.types of Covalent CompoundsEian InganNo ratings yet
- MH1 Che101 CB10 S2019Document262 pagesMH1 Che101 CB10 S2019Hazrat AliNo ratings yet
- Hydrogen Bonding Applications: By-Nikhil Sharma MD Azharuddin YashDocument24 pagesHydrogen Bonding Applications: By-Nikhil Sharma MD Azharuddin YashHaslimi HassanNo ratings yet
- Chapter 2 Ion-Dipole Interaction CKHDocument19 pagesChapter 2 Ion-Dipole Interaction CKHLộc NguyễnNo ratings yet
- (Template) M2 ActivitiesDocument12 pages(Template) M2 ActivitiesRyan Christopher MatosaNo ratings yet
- Unit 1 Module 1 Forces of AttractionDocument9 pagesUnit 1 Module 1 Forces of AttractionRovina Narayan DiasNo ratings yet
- INSTRUCTION: Complete The Table by Computing For The Electronegativity, and Identifying What Is ThereDocument2 pagesINSTRUCTION: Complete The Table by Computing For The Electronegativity, and Identifying What Is ThereChelsea Glaze BarramedaNo ratings yet
- Lecture 7.1 - Inter Molecular ForcesDocument38 pagesLecture 7.1 - Inter Molecular ForcesAdamNo ratings yet
- Structures and Naming Parte 1Document8 pagesStructures and Naming Parte 1Viviana PlacentinoNo ratings yet
- First Mastery Examination in General Chemistry 2Document4 pagesFirst Mastery Examination in General Chemistry 2Mark Christian GeneralaoNo ratings yet
- Forces of Attraction Notes and Practice QuestionsDocument10 pagesForces of Attraction Notes and Practice QuestionsDianna WattNo ratings yet
- Weak InteractionsDocument9 pagesWeak InteractionsShridevi RaviNo ratings yet
- Properties of Ionic and Covalent CompoundsDocument14 pagesProperties of Ionic and Covalent CompoundsNhoj Kram AlitnacnosallivNo ratings yet
- Hydrogen BondingDocument3 pagesHydrogen Bondingdjjagu908No ratings yet
- Kelly Nolan - Electronegativityworksheet1Document2 pagesKelly Nolan - Electronegativityworksheet1Kelly NolanNo ratings yet
- Polar Bonds&Molecules V2Document9 pagesPolar Bonds&Molecules V2vlattaetaeNo ratings yet
- Lecture05-06 Forces Acids BasesDocument59 pagesLecture05-06 Forces Acids BasesLeslieLooNo ratings yet
- Physical Science Smile 2Document12 pagesPhysical Science Smile 2DYLANNo ratings yet
- Worksheet: Polar Bears & Penguins - Electronegativity: NAMEDocument3 pagesWorksheet: Polar Bears & Penguins - Electronegativity: NAMERaymond LiuNo ratings yet
- Lecture SlidesDocument32 pagesLecture Slidesabdulqader.nizarNo ratings yet
- Organic Chemistry One: Bonding and StructureDocument45 pagesOrganic Chemistry One: Bonding and StructureДууяа Б.No ratings yet
- Medicinal Chemistry: Lectures Note 3Document7 pagesMedicinal Chemistry: Lectures Note 3nosaybaNo ratings yet
- Polar and Nonpolar Covalent BondsDocument19 pagesPolar and Nonpolar Covalent BondsJhudy PhotNo ratings yet
- Intermolecular Forces: Liquids, Solids, and Phase ChangesDocument57 pagesIntermolecular Forces: Liquids, Solids, and Phase ChangesB13, Jerex MaxilumNo ratings yet
- 1.4 J-Difusividad en Sólidos PorososDocument7 pages1.4 J-Difusividad en Sólidos PorososDaniel Eduardo ValenzuelaNo ratings yet
- Disolbatzaile Ez UrtsuakDocument27 pagesDisolbatzaile Ez UrtsuakAlazne VegaNo ratings yet
- Polarity & Electronegativity Worksheet SOLVEDDocument1 pagePolarity & Electronegativity Worksheet SOLVEDLili0% (1)
- Physical Science WorksheetDocument2 pagesPhysical Science WorksheetMary GoresNo ratings yet
- 4.2 Electronegativity KEY 2glvvqzDocument2 pages4.2 Electronegativity KEY 2glvvqzAbrogena, Daniela Adiel A.No ratings yet
- 1sdg3y7p9 - AdGE Module 12-Rev Covalent CompoundsDocument15 pages1sdg3y7p9 - AdGE Module 12-Rev Covalent CompoundsJohn RomasantaNo ratings yet
- WKST IMFDocument3 pagesWKST IMFPhilip PrasadNo ratings yet
- Worksheet15 Imf KeyDocument4 pagesWorksheet15 Imf KeyBill alfonsoNo ratings yet
- 4.7 Intermolecular ForcesDocument5 pages4.7 Intermolecular ForcesrachpNo ratings yet
- Chemprincch16 8eDocument46 pagesChemprincch16 8e張芷芸No ratings yet
- Week 2 Polarity of Molecules and Its PropertiesDocument38 pagesWeek 2 Polarity of Molecules and Its Propertieslily smithNo ratings yet
- 6.3 Chemsheets-AS-1016-Forces-between-moleculesDocument3 pages6.3 Chemsheets-AS-1016-Forces-between-moleculesLaura Liu0% (1)
- IB Bonding Note Cards SL HLDocument59 pagesIB Bonding Note Cards SL HL陳定均No ratings yet
- C Chemical BondingDocument33 pagesC Chemical BondingMohitNo ratings yet
- S1-P3c Intermolecular ForcesDocument45 pagesS1-P3c Intermolecular ForcesClifford ChenNo ratings yet
- WHWWHDJDocument2 pagesWHWWHDJChelsea Glaze BarramedaNo ratings yet
- Chapter 4 Part 2Document39 pagesChapter 4 Part 2Rafiya Abdul AzizNo ratings yet
- Chemicals of Life - StudDocument168 pagesChemicals of Life - StudAbdul RahmanNo ratings yet
- Kinetic Molecular Model of Solids and LiquidsDocument5 pagesKinetic Molecular Model of Solids and LiquidsJohn Ahron BalinoNo ratings yet
- 3.0 Chemical BondingDocument27 pages3.0 Chemical BondingTafadzwa MachongweNo ratings yet
- Solubility of Organic CompoundsDocument3 pagesSolubility of Organic CompoundsdeleonmatthewreiNo ratings yet
- Physical Science Modules Week 2Document6 pagesPhysical Science Modules Week 2RODJHEN ANNE P. BARQUILLANo ratings yet
- Weak InteractionsDocument9 pagesWeak InteractionsLovely yadavNo ratings yet
- Activity 2 Polarity of MoleculesDocument2 pagesActivity 2 Polarity of MoleculesRussel LaporeNo ratings yet
- Polarity of Molecules and Its PropertiesDocument34 pagesPolarity of Molecules and Its PropertiesNica Floresta - MendozaNo ratings yet
- CH 11 Intermolecular Attractions and The Properties of Liquids and SolidsDocument69 pagesCH 11 Intermolecular Attractions and The Properties of Liquids and SolidsZenonissya GalwanNo ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular StructureRao GootleyNo ratings yet
- Inorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionFrom EverandInorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- Creative PhotographyDocument2 pagesCreative PhotographyCharlie PuthNo ratings yet
- Community EngagementDocument3 pagesCommunity EngagementCharlie PuthNo ratings yet
- Science 10 Portfolio of Outputs CoverpageDocument1 pageScience 10 Portfolio of Outputs CoverpageCharlie PuthNo ratings yet
- Reviewer TRENDS Lesson 1Document2 pagesReviewer TRENDS Lesson 1Charlie PuthNo ratings yet
- Work Immersion Activity 2Document2 pagesWork Immersion Activity 2Charlie PuthNo ratings yet
- Grade 10 2023 Evaluation Form RecollectionDocument2 pagesGrade 10 2023 Evaluation Form RecollectionCharlie PuthNo ratings yet
- Activity 2Document2 pagesActivity 2Charlie PuthNo ratings yet
- Poem Escapade of TimeDocument2 pagesPoem Escapade of TimeCharlie PuthNo ratings yet
- Physci Answer SheetDocument1 pagePhysci Answer SheetCharlie PuthNo ratings yet
- Physical Science Chapter 01-1-3Document62 pagesPhysical Science Chapter 01-1-3Charlie PuthNo ratings yet
- 3rd Q Music10Document23 pages3rd Q Music10Charlie Puth100% (1)
- Tle DictionaryDocument1 pageTle DictionaryCharlie PuthNo ratings yet
- Essay On Why This Syndrome Is ImportantDocument2 pagesEssay On Why This Syndrome Is ImportantCharlie PuthNo ratings yet
- DaneeeDocument2 pagesDaneeeCharlie PuthNo ratings yet
- Unit 4 Employee SelectionDocument10 pagesUnit 4 Employee SelectionCharlie PuthNo ratings yet
- 11 - HealthDocument14 pages11 - HealthCharlie PuthNo ratings yet
- INTERVIEW CONSENT FORM Non GraduateDocument5 pagesINTERVIEW CONSENT FORM Non GraduateCharlie PuthNo ratings yet
- Unit 2 Indigenous PeoplesDocument12 pagesUnit 2 Indigenous PeoplesCharlie PuthNo ratings yet
- Paper InterviewDocument1 pagePaper InterviewCharlie PuthNo ratings yet
- Family in SocietyDocument17 pagesFamily in SocietyCharlie PuthNo ratings yet
- Seatwork 1Document1 pageSeatwork 1Charlie PuthNo ratings yet
- ResearchPaper AHHAHSDocument20 pagesResearchPaper AHHAHSCharlie PuthNo ratings yet
- Nat Reviewer For EnglishDocument2 pagesNat Reviewer For EnglishCharlie Puth100% (2)
- Unit 3 RecruitmentDocument8 pagesUnit 3 RecruitmentCharlie PuthNo ratings yet
- ACTIVITY 1 - Prelim Output Based-Exam - SuyomDocument2 pagesACTIVITY 1 - Prelim Output Based-Exam - SuyomCharlie PuthNo ratings yet
- Lesson January 2023 1st WeekDocument25 pagesLesson January 2023 1st WeekCharlie PuthNo ratings yet
- Unit 2 HR Planning and Job AnalysisDocument18 pagesUnit 2 HR Planning and Job AnalysisCharlie PuthNo ratings yet
- Module 3 Sociological and and The Study of SocietyDocument6 pagesModule 3 Sociological and and The Study of SocietyCharlie PuthNo ratings yet
- 10 - EducationDocument11 pages10 - EducationCharlie PuthNo ratings yet
- Advocacy SpeechDocument1 pageAdvocacy SpeechCharlie PuthNo ratings yet