Professional Documents
Culture Documents
Rate Expression - FactRecall
Rate Expression - FactRecall
Uploaded by
Ahana KatyalOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rate Expression - FactRecall
Rate Expression - FactRecall
Uploaded by
Ahana KatyalCopyright:
Available Formats
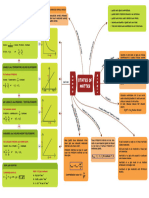
The Rate Expression
Define rate of a reaction
→
The rate of reaction of
a is the
change in concentration
any reactants or
products over time .
I#
The Rate
a - -- -
Expression
- so
,
%,
"
The rate equation is an
experimentally determined relationship .
The constant the of the reaction to the
→ rate is the
proportionality constant
relating rate
concentration of reactants .
→ The order of a reaction is the
power to which the concentration of that
species is raised in the
rate
expression .
Orders of
" " " "on with respect to
rate = K [ A ]m< [ pgynt reactants A and B
rate constant
Determining the Rate Equation
""""" """" "x x x x x ,
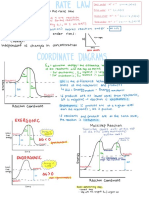
Graphs of Rate
Against concentration
* * ↳
Zero order
É É first order É
order greater
than one
• A *
[ A] [A ] [ A]
The Initial Rate Method
Why ? By measuring the initial rate , the concentrations of all substances in the reaction known at this
-
mixture
•
are
time .
What's for ?
it
Comparing initial concentration and initial rates for of experiments the order with
-
allow
•
the respect to
pairs
each reactant to be found
You might also like
- Electroplating SeminarDocument22 pagesElectroplating SeminarMahalakshmi SahasranamanNo ratings yet
- Emailing Chemical Kinetics (Class 12)Document12 pagesEmailing Chemical Kinetics (Class 12)roceniNo ratings yet
- Physics Short Notes (Part 2)Document20 pagesPhysics Short Notes (Part 2)Suryansh SinghNo ratings yet
- Heinemann Solutions 3 ADocument114 pagesHeinemann Solutions 3 Aannabel28100% (2)
- Approximate Convective-Heating Equations For Hypersonic FlowsDocument7 pagesApproximate Convective-Heating Equations For Hypersonic FlowsIbraheem AlQadi100% (1)
- Emailing Chemical Kinetics (Class 12)Document12 pagesEmailing Chemical Kinetics (Class 12)Bakul ShrivastavaNo ratings yet
- B 49 - 98 R04 - QJQ5 PDFDocument7 pagesB 49 - 98 R04 - QJQ5 PDFOh No PotatoNo ratings yet
- Physics Short Notes 2Document20 pagesPhysics Short Notes 2roceni100% (1)
- CFX Intro 12.0 WS4 Porous MediaDocument14 pagesCFX Intro 12.0 WS4 Porous MediaZavaleta RedlerNo ratings yet
- Chemistry - Kadar TindakbalasDocument40 pagesChemistry - Kadar Tindakbalasrashifah100% (1)
- Soap Production From Waste Cooking Oil PDFDocument72 pagesSoap Production From Waste Cooking Oil PDFBizuye Shetie0% (1)
- What Is PCC (Precipitated Calcium Carbonat)Document5 pagesWhat Is PCC (Precipitated Calcium Carbonat)FahriNo ratings yet
- Chemical Kinetics: Col Lisi On Effective CollisDocument1 pageChemical Kinetics: Col Lisi On Effective ColliseliyachrisNo ratings yet
- Reducedformtonidisedfoomofo - TT: Redox ReactionDocument1 pageReducedformtonidisedfoomofo - TT: Redox ReactionHems MadaviNo ratings yet
- Cheat SheetDocument1 pageCheat Sheetkiarabravo1226No ratings yet
- PhysicsDocument12 pagesPhysicsCan I get 20 subs before 2022No ratings yet
- Half LifeDocument1 pageHalf LifeMelvin SotoNo ratings yet
- Chemical Equilbrium 2Document4 pagesChemical Equilbrium 2bisenpallavi80No ratings yet
- DC CircuitsDocument20 pagesDC CircuitsTaha AzizNo ratings yet
- ChemistryDocument1 pageChemistrycallulafebNo ratings yet
- Unit 3Document10 pagesUnit 3Leen Al-FouzanNo ratings yet
- Driver1985Document9 pagesDriver1985Rishi TenaNo ratings yet
- Chemical Kinetics Class - 1 (Notes)Document26 pagesChemical Kinetics Class - 1 (Notes)ᴜsʜɴᴇᴇᴋNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsAditya PandeyNo ratings yet
- Formula SheetsDocument5 pagesFormula SheetsAayesha NoorNo ratings yet
- QM Formula Sheets by JahanzaibDocument6 pagesQM Formula Sheets by JahanzaibBasit MehrNo ratings yet
- Correlation Regression - Summary NotesDocument4 pagesCorrelation Regression - Summary NotesRockyNo ratings yet
- 1.-Correlational-Analysis-1-of-2 3Document1 page1.-Correlational-Analysis-1-of-2 3Precious Jewel EstoestaNo ratings yet
- Chemical Kinetics Mind MapDocument1 pageChemical Kinetics Mind MapSamridhi MoudgilNo ratings yet
- Chemical KineticsDocument1 pageChemical KineticsSamNo ratings yet
- 2.8 Linear Approximations and DifferentialsDocument7 pages2.8 Linear Approximations and Differentialskhyang0510No ratings yet
- Motion in A Plane PDFDocument48 pagesMotion in A Plane PDFKanhaiya Kumar JhaNo ratings yet
- Non-Isothermal Reactor DesignDocument5 pagesNon-Isothermal Reactor Designnorpius7754No ratings yet
- Final ReviewDocument5 pagesFinal ReviewAn Nguyen Thai HongNo ratings yet
- 11 - 4 - p12 - Minimum Distance Between Can ModesDocument6 pages11 - 4 - p12 - Minimum Distance Between Can Modessiavash mollayiNo ratings yet
- CorrelationDocument16 pagesCorrelationyolozNo ratings yet
- A5. States of MatterDocument1 pageA5. States of MatterHitesh KumarNo ratings yet
- States of MatterDocument1 pageStates of MattergnanavishaljonnalagaddaNo ratings yet
- Chemical KineticsDocument2 pagesChemical KineticsMelvin SotoNo ratings yet
- States of MatterDocument1 pageStates of MatterMalola EducationNo ratings yet
- 2.correlation Regression Summary Notes by Pranav Popat 1Document4 pages2.correlation Regression Summary Notes by Pranav Popat 1Finu x FinuNo ratings yet
- Qdoc - Tips - Astm d523 08 Standard Test Method For Specular GloDocument5 pagesQdoc - Tips - Astm d523 08 Standard Test Method For Specular GlofaizNo ratings yet
- The Time Derivative: Direct Numerical Simulations of Multiphase Flows-3Document5 pagesThe Time Derivative: Direct Numerical Simulations of Multiphase Flows-3Ricardo ACNo ratings yet
- Upd C11 Phy EngDocument23 pagesUpd C11 Phy EngArinjoy Mervyn GomesNo ratings yet
- Ideal GasesDocument13 pagesIdeal Gasesichiwaaa sanNo ratings yet
- Electricity and Magnetism 9Document1 pageElectricity and Magnetism 9Julia KarakiNo ratings yet
- Thermodynamics and Phase BehaviorDocument2 pagesThermodynamics and Phase Behaviorsaurabh pandeyNo ratings yet
- Created by James Riddle With Guidance From Dr. Matthew T. BalhoffDocument2 pagesCreated by James Riddle With Guidance From Dr. Matthew T. BalhoffChrisNo ratings yet
- Week10 5.1 5.3Document29 pagesWeek10 5.1 5.3정승안No ratings yet
- Worked Examples of Divergence EvaluationDocument1 pageWorked Examples of Divergence EvaluationRubensVilhenaFonsecaNo ratings yet
- Relation Relation: Set of Ordered PairsDocument12 pagesRelation Relation: Set of Ordered PairsFerrolinoLouieNo ratings yet
- FormulasDocument3 pagesFormulastemik200303No ratings yet
- Summary of Tests For ConvergenceDocument1 pageSummary of Tests For ConvergenceAurelio ReyesNo ratings yet
- H01 - Mole Balances - BKDocument3 pagesH01 - Mole Balances - BKhsieglerNo ratings yet
- Chapter 2 Kinetics Batch Reactor BKEL FullDocument61 pagesChapter 2 Kinetics Batch Reactor BKEL Fulldhuy2399No ratings yet
- Chapter 3 - Part 3Document7 pagesChapter 3 - Part 3Thevhan MurallyNo ratings yet
- Français Français: o o o o o o o oDocument18 pagesFrançais Français: o o o o o o o oTakieddine BenlahcenNo ratings yet
- Potential Energy SurfaceDocument21 pagesPotential Energy SurfaceSIDDHARTH MARATHANo ratings yet
- Chapter (5678) - (p1 2) PDFDocument2 pagesChapter (5678) - (p1 2) PDFHyunSung KimNo ratings yet
- Linear Correlation CoefficientDocument4 pagesLinear Correlation Coefficientkaycelyn jimenezNo ratings yet
- Olocity: FluidDocument75 pagesOlocity: FluidERRNo ratings yet
- P3 Motion in A Straight LineDocument1 pageP3 Motion in A Straight LineberburnleyNo ratings yet
- Maths Important PointsDocument146 pagesMaths Important Pointsgspareek1111No ratings yet
- Lecture 8 and 9 Regression Correlation and IndexDocument32 pagesLecture 8 and 9 Regression Correlation and Indexআসিফ বিন মনজুরNo ratings yet
- Chapter 7 Correlation and Regression Lyst5582Document13 pagesChapter 7 Correlation and Regression Lyst5582dauzisohail1999No ratings yet
- Hough - The Crystallography, Metallography and Composition of Gold PDFDocument6 pagesHough - The Crystallography, Metallography and Composition of Gold PDFSantiaGoAlejandRoNo ratings yet
- Halogen DerivativeDocument32 pagesHalogen Derivativesachin pantNo ratings yet
- 2021 CHE 2231 WK6 Sub Elimination Worksheet-1Document15 pages2021 CHE 2231 WK6 Sub Elimination Worksheet-1dancer88838No ratings yet
- Assignment 4Document3 pagesAssignment 4Đạt Trương MinhNo ratings yet
- Periodicity: Chemistry For Engineers (CH011IU) - Lecture 05 - Semester 1: 2021-2022 1Document50 pagesPeriodicity: Chemistry For Engineers (CH011IU) - Lecture 05 - Semester 1: 2021-2022 1Thanhh ThaooNo ratings yet
- Stabilization of Soil Using Bio-Enzyme (Terrazyme) : Gogireddy SomireddyDocument9 pagesStabilization of Soil Using Bio-Enzyme (Terrazyme) : Gogireddy SomireddyFARY MARIYAMNo ratings yet
- Radiation Safety SOP: Scope/PurposeDocument4 pagesRadiation Safety SOP: Scope/Purposezen AlkaffNo ratings yet
- Wave Motion PhysicsDocument24 pagesWave Motion PhysicsmakmgmNo ratings yet
- Exergy Analyses: Nmb-21303 Thermodinamik IDocument26 pagesExergy Analyses: Nmb-21303 Thermodinamik ISafwan SolihinNo ratings yet
- List of Laboratory Apparatus and Their UsesDocument7 pagesList of Laboratory Apparatus and Their UsesNhorain A. Ariman-MayoNo ratings yet
- Chapter 13 Heat TransferDocument18 pagesChapter 13 Heat TransferaimanrslnNo ratings yet
- Free PDF Pharmaceutical Experimental Design and Interpretation N A Armstrong and K C JamesDocument2 pagesFree PDF Pharmaceutical Experimental Design and Interpretation N A Armstrong and K C JamesBrentNo ratings yet
- Exploring Liminal Spaces by SlidesgoDocument53 pagesExploring Liminal Spaces by SlidesgoregindewindaaNo ratings yet
- Laboratory Exercise 3 - AdU1sAY2223 - ANIESDocument2 pagesLaboratory Exercise 3 - AdU1sAY2223 - ANIESRhean Rheign VergaraNo ratings yet
- Electro Positive Nature, Metallic and Non-Metallic Nature, Acidic and Basic Nature of OxidesDocument5 pagesElectro Positive Nature, Metallic and Non-Metallic Nature, Acidic and Basic Nature of OxidesAaditya AgrahariNo ratings yet
- Fischer-Tropsh Energy Balance IMCCRE (2904)Document2 pagesFischer-Tropsh Energy Balance IMCCRE (2904)chuertaNo ratings yet
- Alkohol Tata NamaDocument4 pagesAlkohol Tata NamaIkke SantikaNo ratings yet
- Chemical Constituents and Antibacterial Activities of Leaves of Sumatran King Fern (Angiopteris Evecta G. Forst HOFFM.)Document8 pagesChemical Constituents and Antibacterial Activities of Leaves of Sumatran King Fern (Angiopteris Evecta G. Forst HOFFM.)ElfiaNeswitaNo ratings yet
- FMC - Novolastic Subsea Thermal Insulation - LOW RESDocument3 pagesFMC - Novolastic Subsea Thermal Insulation - LOW RESalphading50% (2)
- Generalized Single-Degree-of-Freedom Systems: Chopra: Prentice-Hall PAGES JUL. 19, 2000 14:21 ICC Oregon (503) 221-9911Document38 pagesGeneralized Single-Degree-of-Freedom Systems: Chopra: Prentice-Hall PAGES JUL. 19, 2000 14:21 ICC Oregon (503) 221-9911MakaraSoyNo ratings yet
- Kuliah Geofluida #5 Transportasi MassaDocument48 pagesKuliah Geofluida #5 Transportasi Massafazrie akbar0% (1)
- Lecture 08Document4 pagesLecture 08RajeevNo ratings yet
- WRITTEN WORK 2 - Attempt Review Chem122Document6 pagesWRITTEN WORK 2 - Attempt Review Chem122Lymenson Boongaling100% (2)
- HYDRAULIC MODEL INVENTORY (Pipe) OF DMA-1 (Rairangpur)Document36 pagesHYDRAULIC MODEL INVENTORY (Pipe) OF DMA-1 (Rairangpur)Abhishek PandaNo ratings yet