Professional Documents
Culture Documents
C5 - Reactions of Acids
C5 - Reactions of Acids
Uploaded by
Alfie MurrayOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C5 - Reactions of Acids
C5 - Reactions of Acids
Uploaded by
Alfie MurrayCopyright:
Available Formats
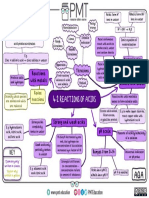
C5 – Reactions Of Acids
Metal oxides and metal hydroxides dissolve in water. These soluble compounds
are called alkalis. Bases wont dissolve in water
Any extra notes go here: Any extra notes go here:
Acid + Metal Oxide -> Salt + Water Acid + Metal Carbonate -> Salt + Water + Carbon Dioxide
Acid + Metal Hydroxide -> Salt + Water Metal Carbonates are also bases. They react acids to produce a salt, water,
Salt produces depends on the acid used, and carbon dioxide.
E.g. Hydrochloric acid + copper oxide -> copper You can make soluble salts using an insoluble base.
chloride + water.
About The Figure
Acids take part in reactions in which salts are
produced. In these reactions, the
hydrogen ions in the acids are replaced by
metal ions.
Extra Notes
1)Use the pipette and pipette filler to add a measured volume of sodium
hydroxide solution to a clean conical flask. 2)Add a few drops of indicator
and put the conical flask on a white tile. 3)Fill the burette with
hydrochloric acid and note the starting volume. 4)Slowly add the acid from
the burette to the alkali in the conical flask, swirling to mix. 5)Stop adding
the acid when the endpoint is reached (when the indicator first permanently
changes colour). Note the final volume reading. 6)Repeat steps 1 to 5
until concordant titres are obtained. More accurate results are obtained if
acid is added drop by drop near to the endpoint.
Key Points Key Words
- A salt is a compound formed when the hydrogen in an acid is wholly or - Titration
partially replaced by metal or ammonium ions. - Acid
- Salts can be made by reacting a suitable metal with an acid. The metal - Alkali
must be above hydrogen in the reactivity series, but not dangerously - Metals
reactive. - Salt
- The reaction between a metal and an acid produces hydrogen gas as well - Water
as a salt. A sample of the salt made can then be crystallised out of - Carbon
solution by evaporating off the water. Dioxide
- 4 The reaction between a metal and an acid is an example of a redox
reaction. The metal atoms lose electrons and are oxidised, and hydrogen
ions from the acid gain electrons and are reduced.
You might also like
- (Ebook - PDF - Chemistry) Methamphetamine SynthesisDocument4 pages(Ebook - PDF - Chemistry) Methamphetamine SynthesisJOne Nakasamai33% (6)
- Metals and Non-MetalsDocument20 pagesMetals and Non-MetalsDevyansh MishraNo ratings yet
- Sample Questions - Chapter 22Document4 pagesSample Questions - Chapter 22Rasel IslamNo ratings yet
- Use of Timber As Construction MaterialsDocument40 pagesUse of Timber As Construction MaterialsIfiokobong Akpan100% (1)
- Chemistry Notes (Acids, Bases and Salts)Document3 pagesChemistry Notes (Acids, Bases and Salts)Teo Jia Ming Nickolas92% (13)
- 3.1 The Reactivity Series of MetalsDocument17 pages3.1 The Reactivity Series of MetalsWafa OsmanNo ratings yet
- Chapter 2 - Acids, Bases and Salts: Class - X ScienceDocument14 pagesChapter 2 - Acids, Bases and Salts: Class - X ScienceDhruv AsodariaNo ratings yet
- Preparing Common Salts G8Document21 pagesPreparing Common Salts G8shanaayaa kunder100% (1)
- Acids and Bases NotesDocument10 pagesAcids and Bases NotesThaarvena RetinaNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- All Alkaloids Final 2015 GeneralDocument65 pagesAll Alkaloids Final 2015 GeneralMourad NawarNo ratings yet
- Pacop Violet 1Document476 pagesPacop Violet 1MA. CHARMIA SAMATRANo ratings yet
- Water Technology Understanding, Interpreting and UTILIZING WATER ANALYSIS DATADocument12 pagesWater Technology Understanding, Interpreting and UTILIZING WATER ANALYSIS DATAMohamed HaboNo ratings yet
- Acids and BasesDocument98 pagesAcids and BasesLaziNo ratings yet
- Alchemy IlluminatedDocument37 pagesAlchemy IlluminatedAyuxoç ÀsrengNo ratings yet
- Corrosion in The Crude Distillation Unit Overhead Line: Contributors and SolutionsDocument16 pagesCorrosion in The Crude Distillation Unit Overhead Line: Contributors and SolutionsMaría Alejandra Quintero PinillaNo ratings yet
- MT 165 Ultraviolet Absorption Test For Evaluation of Ethylenebis (Dithiocarbamate)Document29 pagesMT 165 Ultraviolet Absorption Test For Evaluation of Ethylenebis (Dithiocarbamate)Felipe NavarreteNo ratings yet
- Acids Bases and SaltsDocument6 pagesAcids Bases and SaltsHanaa AbouziedNo ratings yet
- Acid, Bases and Salts.Document14 pagesAcid, Bases and Salts.lucy.murrayNo ratings yet
- Presentasi Science Grup 1Document12 pagesPresentasi Science Grup 1Chelsica ChelsicaNo ratings yet
- Acid Bases & SaltsDocument7 pagesAcid Bases & Saltssհízα ƒαlαҡNo ratings yet
- Class 10 Science Chapter NotesDocument192 pagesClass 10 Science Chapter NotesTarunNo ratings yet
- Acid Base and SaltsDocument45 pagesAcid Base and SaltsPankaj KumarNo ratings yet
- Making Salts: Neutralisation ReactionsDocument4 pagesMaking Salts: Neutralisation ReactionsPedro Moreno de SouzaNo ratings yet
- NOTES - Acids, Bases and SaltsDocument31 pagesNOTES - Acids, Bases and SaltsKartik ChauhanNo ratings yet
- Acids, Bases and SaltsDocument25 pagesAcids, Bases and SaltsAnthonya KnightNo ratings yet
- Acids BasesDocument25 pagesAcids BasesNermin AkberovaNo ratings yet
- Acids, Bases IXDocument10 pagesAcids, Bases IXSukaina hussainNo ratings yet
- Acid and BasesDocument80 pagesAcid and BasesMenaga IlangkovanNo ratings yet
- Acids: Common Acids We Come Across EverydayDocument5 pagesAcids: Common Acids We Come Across EverydayFarzan ButtNo ratings yet
- Grade 10 Chemistry Week 12 Lesson 1Document4 pagesGrade 10 Chemistry Week 12 Lesson 1nesiaroberts903No ratings yet
- ACIDS and BASES Notes & WorksheetDocument9 pagesACIDS and BASES Notes & WorksheetAdeenaNo ratings yet
- Oxides and Its TypesDocument21 pagesOxides and Its TypesnamrataNo ratings yet
- Example of Organic AcidsDocument7 pagesExample of Organic AcidsAUDREYNo ratings yet
- Chemistr Y Notes: - Solencia HamiltonDocument56 pagesChemistr Y Notes: - Solencia HamiltonManushka ThomasNo ratings yet
- AC RX of OxidesDocument4 pagesAC RX of OxidesNancy MohamedNo ratings yet
- Chem Acids, Bases and SaltsDocument27 pagesChem Acids, Bases and SaltsJun ZheNo ratings yet
- Zaim NotesDocument5 pagesZaim NotesZafirah SuffianNo ratings yet
- Chem - Acids and Bases and Ionic EquationsDocument23 pagesChem - Acids and Bases and Ionic EquationsYasser AliNo ratings yet
- 4.2. Reactions of AcidsDocument1 page4.2. Reactions of AcidsAnais BegueNo ratings yet
- Acids, Bases, Salts-IG ChemistryDocument16 pagesAcids, Bases, Salts-IG ChemistryRashi GhadiyaNo ratings yet
- Acids, Bases & Salts: IndicatorsDocument7 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Chem ReviewDocument3 pagesChem Reviewichika lymNo ratings yet
- Salt PreparationDocument41 pagesSalt Preparationsidsolegend123No ratings yet
- Naming Salts: Salt Compound Neutralisation Acid BaseDocument5 pagesNaming Salts: Salt Compound Neutralisation Acid BaseErwin Mauricio Padilla RocaNo ratings yet
- Chem Notes 3e MR Machipanda Term 2Document22 pagesChem Notes 3e MR Machipanda Term 2Tendai MugengeNo ratings yet
- Acids, Alkalis and Salts: Acids Are Substances That Form Hydrogen Ions, HDocument1 pageAcids, Alkalis and Salts: Acids Are Substances That Form Hydrogen Ions, Hsagayaamitha7388No ratings yet
- Acids, Bases and SaltsDocument5 pagesAcids, Bases and SaltsIsa ShahidNo ratings yet
- Acid Bases and Salts 2022-23Document8 pagesAcid Bases and Salts 2022-23Yasha RizviNo ratings yet
- Acids, Bases & Salts: IndicatorsDocument4 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Chemistry Revision Notes For O LevelDocument28 pagesChemistry Revision Notes For O LevelshinNo ratings yet
- Acids and BasesDocument7 pagesAcids and BasesM.zuhair asifNo ratings yet
- ( (Chapter 8&9 - Acids and Bases, Salts) )Document8 pages( (Chapter 8&9 - Acids and Bases, Salts) )bharadiadishitaNo ratings yet
- CH-11 Acids, Bases and Salts NotesDocument4 pagesCH-11 Acids, Bases and Salts NotesAnish KanthetiNo ratings yet
- Acids, Bases and SaltsDocument28 pagesAcids, Bases and SaltsFavour Emehibe-AmaechiNo ratings yet
- Making Salts NotesDocument17 pagesMaking Salts NotesLola AdegbonmireNo ratings yet
- Bases Acids: Strong Acid Strong BaseDocument1 pageBases Acids: Strong Acid Strong Baseshrikant raiNo ratings yet
- SALTSDocument4 pagesSALTSfatimawaqar657No ratings yet
- Revision Notes On Acids, Bases and SaltsDocument3 pagesRevision Notes On Acids, Bases and SaltsVikas SharmaNo ratings yet
- Chemistry Acids and Bases Research SheetDocument2 pagesChemistry Acids and Bases Research SheetSheri-Ann AshmanNo ratings yet
- CLASS X - CHEM - CH 2 Day 2 - Chemical Properties of AcidsDocument4 pagesCLASS X - CHEM - CH 2 Day 2 - Chemical Properties of Acidsgourav kaliaNo ratings yet
- Acids, Bases, Salts - 1Document9 pagesAcids, Bases, Salts - 1Ashleigh JarrettNo ratings yet
- Acids and BasesDocument7 pagesAcids and BasesaquamogolwaneNo ratings yet
- X - SM - Sci - Acid Bases SaltsDocument14 pagesX - SM - Sci - Acid Bases SaltsKabir AroraNo ratings yet
- Chemistry 10Document29 pagesChemistry 10Javed QasimNo ratings yet
- A Closer Look at Acids: Chapter - 9.2Document13 pagesA Closer Look at Acids: Chapter - 9.2goodboyhokyaNo ratings yet
- Acid and Bases 2Document5 pagesAcid and Bases 2liyasariNo ratings yet
- Acids and Bases: MD - Safwat X Riesaf HossainDocument9 pagesAcids and Bases: MD - Safwat X Riesaf HossainMd SafwatNo ratings yet
- Acids Bases SaltsDocument27 pagesAcids Bases SaltsDarwisy SyazwanNo ratings yet
- Inorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionFrom EverandInorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- How to Do Chemical Tricks: Containing Over One Hundred Highly Amusing and Instructive Tricks With ChemicalsFrom EverandHow to Do Chemical Tricks: Containing Over One Hundred Highly Amusing and Instructive Tricks With ChemicalsNo ratings yet
- Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International General Certificate of Secondary EducationAprillia YapNo ratings yet
- Main Group Oganometallics: Shriver and Atkins, Chapter 15Document24 pagesMain Group Oganometallics: Shriver and Atkins, Chapter 15José Augusto VillarNo ratings yet
- WAEC Chemistry Syllabus 2022 PDF Download AvailableDocument26 pagesWAEC Chemistry Syllabus 2022 PDF Download AvailableSamuel AikinsNo ratings yet
- National List of Concessions: China: No H.S. 2005 Description Margin of Preference (%)Document58 pagesNational List of Concessions: China: No H.S. 2005 Description Margin of Preference (%)rithbaan basuNo ratings yet
- ĐỀ SỐ 01-HSG ANH 9 TỈNHDocument16 pagesĐỀ SỐ 01-HSG ANH 9 TỈNHYoon HaNo ratings yet
- In Komp A Tibi LitasDocument54 pagesIn Komp A Tibi LitasHifi Rizki.RNo ratings yet
- Icse Class 10 March21 Chemistry Question Paper With Solutions 2023Document23 pagesIcse Class 10 March21 Chemistry Question Paper With Solutions 2023Ankit KumarNo ratings yet
- SPM Chemistry Formula List Form4Document14 pagesSPM Chemistry Formula List Form4Heng HoweNo ratings yet
- Solubility and Thermodynamic Functions For A 3-2 Salt, Samarium Sulfate, in Water and Sulfuric Acid Solutions at Temperatures To 350°cDocument6 pagesSolubility and Thermodynamic Functions For A 3-2 Salt, Samarium Sulfate, in Water and Sulfuric Acid Solutions at Temperatures To 350°cWalter Sperandio SampaioNo ratings yet
- CTSC Matric Masterclasses Acid and Bases 2020-1Document13 pagesCTSC Matric Masterclasses Acid and Bases 2020-1mxolisi mkhumaneNo ratings yet
- Synthetic Detergents 100 Years of HistoryDocument16 pagesSynthetic Detergents 100 Years of HistoryJayantha TennakoonNo ratings yet
- Assertion and Reason QuestionsDocument11 pagesAssertion and Reason Questionssreejaps45No ratings yet
- Ash-Related Issues in Fluidized-Bed Combustion of BiomassesDocument11 pagesAsh-Related Issues in Fluidized-Bed Combustion of Biomassesduygu esmerNo ratings yet
- GCSE CHEM Past Papers Mark Schemes Standard MayJune Series 2017 21792Document22 pagesGCSE CHEM Past Papers Mark Schemes Standard MayJune Series 2017 21792Brian musyaniNo ratings yet
- Mono58 7Document119 pagesMono58 7Marcus BragaNo ratings yet
- 3 Asam BasaDocument162 pages3 Asam BasaainulavidaNo ratings yet
- Notes On Energy ChangesDocument8 pagesNotes On Energy ChangesHao TanNo ratings yet
- Class X Science Practice Test Ak 2022-23Document15 pagesClass X Science Practice Test Ak 2022-23Tanish MehtaNo ratings yet
- Carbonates and Bicarbonates Explained in Detail 1Document39 pagesCarbonates and Bicarbonates Explained in Detail 1api-271064836No ratings yet