Professional Documents
Culture Documents
Race 1 1675773972
Uploaded by
attackerasp1234Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Race 1 1675773972
Uploaded by
attackerasp1234Copyright:
Available Formats
JEE (Main + Advanced) 2024
JEE (Main + Advanced) 2024
ENTHUSIAST COURSE
ENTHUSIAST COURSE
RACE # 16 PHYSICAL CHEMISTRY
M.M. : 32 TIME : 35 Min.
Single correct :
1. In the presence of a catalyst, the heat evolved or absorbed during the reaction ___________. [3]
(A) increases. (B) decreases.
(C) remains unchanged. (D) may increase or decrease.
2. For a complex reaction ______________. [3]
(A) order of overall reaction is same as molecularity of the slowest step (provided slowest step having no
reaction intermediate)
(B) order of overall reaction is less than the molecularity of the slowest step.
(C) order of overall reaction is greater than molecularity of the slowest step.
(D) molecularity of the slowest step is never zero or non interger.
3. Mark the incorrect statements. [3]
®
(A) Catalyst provides an alternative pathway to reaction mechanism.
(B) Catalyst raises the activation energy.
(C) Catalyst lowers the activation energy.
(D) Catalyst alters enthalpy change of the reaction.
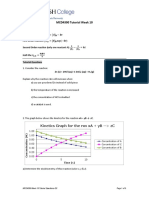
4. A gas is taken isochorically from state A to state C as shown in the graph. Choose the correct statement-
B C
P
A
T
(A)Moles of gas first remains constant and then increases [3]
(B) Moles of gas first increases and then remains constant.
(C) Moles of gas first remains constant and then decreases
(D) Moles of gas first decreases and then remains constant.
5. A closed vessel contains helium and ozone at a pressure of P atm. The ratio of He and oxygen atoms
is 1 : 1. If helium is removed from the vessel, the pressure of the system will reduce to:
(A) 0.5 P atm (B) 0.75 P atm (C) 0.25 P atm (D) 0.33 P atm [3]
6. Consider Figure and mark the correct option. [3]
Activated complex
E1
Energy
Products
E2
Reactants
(A) Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
(B) Activation energy of forward reaction is E1+E2 and product is more stable than reactant.
(C) Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable
than product.
(D) Activation energy of backward reaction is E1 and product is more stable than reactant.
E-26 /37 Physical / R # 01-20 (Booklet)
JEE (Main + Advanced) 2024
ENTHUSIAST COURSE
7. For a gas reaction A P at T (K) the rate is given by [3]
rate = k1 pA2 atm/hr
rate = k2CA2 mol/litre/hr
the relation between k1 and k2 is
(A) k2 = k1 (B) k2 = k1 RT (C) k2 = k1/RT (D) k2 = k1(RT)2
8. For the reactions (i) A
KI
P. [3]
& (ii) B
K II Q, following observation is made.
1M
Concentration
1M
Concentration
®
0.5
[A] 0.5
0.25
[B]
0 30 60
Time (min) 60 Time (min)
KI
Calculate K , where K1 and KII are rate constants for the respective reaction.
II
(A) 2.772 (B) 1 (C) 0.36 (D) None of these
Multiple correct :
9. Consider the Arrhenius equation given below and mark the correct option. k = A e–Ea / RT [3]
(A) Rate constant increases exponentially with increasing activation energy and decreasing temperature.
(B) Rate constant decreases exponentially with increasing activation energy and decreasing temperature.
(C) Rate constant increases exponentially with decreasing activation energy and decreasing temperature.
(D) Rate constant increases exponentially with decreasing activation energy and increasing temperature.
10. tx/y= time in which x/y fraction of reactant converts into product. [5]
ColumnI Column II
(A) t5/9 (P) Equal to 54 sec. if t1/3 is 18 sec. in case of first order reaction.
(B) t19/27 (Q) Equal to 32 sec. if t1/4 is 16 sec. in case of first order reaction.
(C) t7/8 (R) Equal to 56 sec. if t1/3 is 4 sec. in case of second order reaction.
(D) t7/16 (S) Equal to 30 sec. if t1/3 is 18 sec. in case of zero order reaction.
(T) Equal to 28 sec. if t1/2 is 16 sec. in case of zero order reaction.
Physical / R # 01-20 (Booklet) E-27/37
You might also like
- Answer Scheme Practice CORONA - 1-1Document12 pagesAnswer Scheme Practice CORONA - 1-1Mumtaz Barhiya100% (1)
- Che 05012 Chemical KineticsDocument8 pagesChe 05012 Chemical Kineticstri anggraini arifNo ratings yet
- Enzyme NotesDocument4 pagesEnzyme NotesMaulana Makhmud100% (2)
- Hi HelloDocument4 pagesHi Hellotenik68424No ratings yet
- 13 Reaction Kinetics (S)Document32 pages13 Reaction Kinetics (S)Mr TanNo ratings yet
- CPP12 Mixed Reactions-20220809124004923777Document6 pagesCPP12 Mixed Reactions-20220809124004923777Ashutosh SinghNo ratings yet
- Organic ChemistryDocument5 pagesOrganic ChemistryEve LeeNo ratings yet
- Illuminati - 2019: Advanced Chemistry Assignment - 4B - Physical Chemistry Class-XiithDocument12 pagesIlluminati - 2019: Advanced Chemistry Assignment - 4B - Physical Chemistry Class-XiithBiswajit GhoshNo ratings yet
- JEE AssignmentsDocument12 pagesJEE AssignmentsKriti GargNo ratings yet
- Log K ®: Section A //X Choose Correct Answer From The Given Options. (Each Carries 1 Mark)Document20 pagesLog K ®: Section A //X Choose Correct Answer From The Given Options. (Each Carries 1 Mark)WhoaretoNo ratings yet
- Asc 2016Document25 pagesAsc 2016Samruddhi MohiteNo ratings yet
- HN OH: Multiple Choice QuestionsDocument5 pagesHN OH: Multiple Choice QuestionsMahesh Kumar KumawatNo ratings yet
- DPPS-2 - Chemical KineticsDocument2 pagesDPPS-2 - Chemical KineticsShrish PratapNo ratings yet
- Test On Chemical KineticsDocument4 pagesTest On Chemical Kineticsdevansh dewanNo ratings yet
- 2020-21 S.S. Tutorials: Student ID Unit Test: 03 - ADocument3 pages2020-21 S.S. Tutorials: Student ID Unit Test: 03 - ASonuSharmaNo ratings yet
- 1 Homo Test-1 Without AnswerDocument9 pages1 Homo Test-1 Without Answerchiranjeet mishraNo ratings yet
- Class 11 Chemistry Sample PaperDocument6 pagesClass 11 Chemistry Sample PaperDamodar KasukurthiNo ratings yet
- Chemical Kinetics - PYQ - (NSEC)Document5 pagesChemical Kinetics - PYQ - (NSEC)LAKHAN KHANDELWALNo ratings yet
- Exam I Review QuestionsDocument9 pagesExam I Review QuestionsRylan SmolikNo ratings yet
- 31 TT-Poll - C-31 (Chemistry) Chemical KineticsDocument5 pages31 TT-Poll - C-31 (Chemistry) Chemical KineticsNandish PatelNo ratings yet
- DPP-Chemical Equilibrium - CombinedDocument31 pagesDPP-Chemical Equilibrium - CombinedAtharva WatekarNo ratings yet
- General Instructions:: Sample Question Paper - 26 Chemistry (043) Class-XII, Session: 2021-22Document7 pagesGeneral Instructions:: Sample Question Paper - 26 Chemistry (043) Class-XII, Session: 2021-22unique oneNo ratings yet
- 02-Chemical Kinetic - Telegram - @JEE - BOOKSDocument11 pages02-Chemical Kinetic - Telegram - @JEE - BOOKSRdNo ratings yet
- XI Chemistry QPDocument6 pagesXI Chemistry QPuddyan TripathiNo ratings yet
- S-Chemistry-I-4th QBT PDFDocument5 pagesS-Chemistry-I-4th QBT PDFKhubaib AhmedNo ratings yet
- 4 Chemical-Kinetics 500930Document7 pages4 Chemical-Kinetics 500930Ahkil NandaNo ratings yet
- Chemical Kinetics: (Physical Chemistry)Document26 pagesChemical Kinetics: (Physical Chemistry)keshavNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- Solutions 2018 Mains 2Document11 pagesSolutions 2018 Mains 2sudhir_kumar_33No ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Module AG Sir Chemical EquilibriumDocument10 pagesModule AG Sir Chemical EquilibriumArnavNo ratings yet
- Chemisstry FormulaDocument11 pagesChemisstry FormulaSharifah RenahNo ratings yet
- Chemistry QP2Document6 pagesChemistry QP2Jinendra UvarajNo ratings yet
- Topic: Chemical Kinetic: DT) NH (D 2 DT) H (DDocument5 pagesTopic: Chemical Kinetic: DT) NH (D 2 DT) H (Dvictoria schoolNo ratings yet
- S.S. Tutorials 2020-21: Student IDDocument3 pagesS.S. Tutorials 2020-21: Student IDSonuSharmaNo ratings yet
- Chemistry: Crash Course For JEE Main 2020Document17 pagesChemistry: Crash Course For JEE Main 2020QSQFNo ratings yet
- 5 6070954144254919518Document32 pages5 6070954144254919518sujal thawareNo ratings yet
- Chemical Sciences: Paper IIDocument12 pagesChemical Sciences: Paper IIRavikanthNo ratings yet
- 4 - Chemical Kinetics & RadioactivityDocument19 pages4 - Chemical Kinetics & RadioactivityNimeshNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- NAT January SET-2 XI To XIIDocument4 pagesNAT January SET-2 XI To XIIAayush NagpalNo ratings yet
- Home Assignment CHEMISTRYDocument14 pagesHome Assignment CHEMISTRYPriyanka KesharwaniNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Chemical Kinetics PDFDocument23 pagesCBSE Class 12 Chem Notes Question Bank Chemical Kinetics PDFAshika D ChandavarkarNo ratings yet
- Chemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17Document8 pagesChemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17AbhinavNo ratings yet
- Kinetics Review Questions - SolutionsDocument3 pagesKinetics Review Questions - SolutionsMarikNo ratings yet
- Chemical Kinetics PDFDocument5 pagesChemical Kinetics PDFBrahmanand TiwariNo ratings yet
- Bai Tap Chuong Kinetic ReactionDocument5 pagesBai Tap Chuong Kinetic ReactionPHƯƠNG ĐẶNG YẾNNo ratings yet
- Bai Tap Chuong Kinetic ReactionDocument5 pagesBai Tap Chuong Kinetic ReactionPHƯƠNG ĐẶNG YẾNNo ratings yet
- ch-29 Que - Paper PDFDocument36 pagesch-29 Que - Paper PDFkrishnaNo ratings yet
- C Ch-05 ThermodynamicsDocument7 pagesC Ch-05 Thermodynamicsmysoftinfo.incNo ratings yet
- Neet 20Document6 pagesNeet 20h47xa4t5No ratings yet
- Kinetics Test 1Document8 pagesKinetics Test 1Aditya TiwariNo ratings yet
- Chapter7 - CHEMICAL EQUILIBRIUMDocument30 pagesChapter7 - CHEMICAL EQUILIBRIUMadhwa100% (1)
- Chem2exam2 PDFDocument6 pagesChem2exam2 PDFLouis ParrNo ratings yet
- Business Card 9 Oct 2022Document5 pagesBusiness Card 9 Oct 2022Milan KadamNo ratings yet
- Chemical KinematicsDocument3 pagesChemical KinematicsLyra GurimbaoNo ratings yet
- MaterialDocument10 pagesMaterialgudias375No ratings yet
- Thermodynamics AssignmentDocument11 pagesThermodynamics Assignmenthimanshurbz1081No ratings yet
- Kinetics II (Multiple Choice) QPDocument10 pagesKinetics II (Multiple Choice) QPMZWAANo ratings yet
- .Archphys Sciences p2 Gr12 QP Sept2023 - EnglishDocument23 pages.Archphys Sciences p2 Gr12 QP Sept2023 - EnglishZNo ratings yet
- Kinetics Mc1Document6 pagesKinetics Mc1hylee102594No ratings yet
- 43 Class Test (E-ToAS, TNAS, TAAS) - StudentDocument4 pages43 Class Test (E-ToAS, TNAS, TAAS) - Studentattackerasp1234No ratings yet
- Race 1 1675856339Document1 pageRace 1 1675856339attackerasp1234No ratings yet
- Paper 2 With Solution PhysicsDocument17 pagesPaper 2 With Solution Physicsattackerasp1234No ratings yet
- Oc Taas Quiz 6 StudentDocument4 pagesOc Taas Quiz 6 Studentattackerasp1234No ratings yet
- Race 1 1675664085Document1 pageRace 1 1675664085attackerasp1234No ratings yet
- Some Intrinsic Kinetic Equations and Deactivatioll Mechanisms Leading To Deactivation Curves With A Residual Activity Corella1988Document7 pagesSome Intrinsic Kinetic Equations and Deactivatioll Mechanisms Leading To Deactivation Curves With A Residual Activity Corella1988Jose NNo ratings yet
- "Chemical Process Modeling and Simulation": Submitted byDocument54 pages"Chemical Process Modeling and Simulation": Submitted byJay29No ratings yet
- Chemical Kinetics Part IDocument45 pagesChemical Kinetics Part IKarthikanAmirthalingamNo ratings yet
- Luyben 2011Document17 pagesLuyben 2011Paola Plazas Alarcón100% (1)
- 15 Chem KinetDocument51 pages15 Chem KinetRoua Ali100% (2)
- AS LEVEL BIOLOGY Paper 1 EnzymesDocument54 pagesAS LEVEL BIOLOGY Paper 1 EnzymesADEEL AHMADNo ratings yet
- Newman 1968Document16 pagesNewman 1968Gabriel Leonardo Tacchi NascimentoNo ratings yet
- Biocatalysis Questions and AnswersDocument9 pagesBiocatalysis Questions and Answerskumara guruparanNo ratings yet
- Chapter 1 - Chemical Kinetics Part 1Document46 pagesChapter 1 - Chemical Kinetics Part 1NUR DINI MAISARAH BINTI HEZAL / UPMNo ratings yet
- Syllabus 3102 Spring 2018Document5 pagesSyllabus 3102 Spring 2018Arthur LembongNo ratings yet
- CFE - 10 - Chemical Kinetics Part 1Document24 pagesCFE - 10 - Chemical Kinetics Part 1Christian C. SuaseNo ratings yet
- The Base-Catalyzed-Hydrolysis and Condensation-Reactions of Dilute and Concentrated Teos SolutionsDocument7 pagesThe Base-Catalyzed-Hydrolysis and Condensation-Reactions of Dilute and Concentrated Teos SolutionscoloreyeNo ratings yet
- CRE EngineeringDocument19 pagesCRE EngineeringTapajyoti GhoshNo ratings yet
- EnzymeDocument15 pagesEnzymeShreyashNo ratings yet
- Enz FactorsDocument25 pagesEnz FactorsSparrowNo ratings yet
- Martin Schmal (Auth.) - Heterogeneous Catalysis and Its Industrial Applications-Springer International Publishing (2016)Document382 pagesMartin Schmal (Auth.) - Heterogeneous Catalysis and Its Industrial Applications-Springer International Publishing (2016)Rebeca Albino100% (1)
- Enzymes Mind MapDocument1 pageEnzymes Mind Mapruaridh.sayerNo ratings yet
- Chemical Kinetics Question BankDocument5 pagesChemical Kinetics Question BankShivam kumarNo ratings yet
- Modelling and SimulationDocument109 pagesModelling and SimulationReya RaghunathanNo ratings yet
- Chemical Reaction EngineeringDocument11 pagesChemical Reaction Engineeringkushaal narothamNo ratings yet
- Effect of Dissociation On CombustionDocument11 pagesEffect of Dissociation On CombustionBanwari Lal PrajapatNo ratings yet
- EnzymeKinetics PDFDocument21 pagesEnzymeKinetics PDFaathiraNo ratings yet
- Enzymology SyllabusDocument1 pageEnzymology SyllabusKamlesh SahuNo ratings yet
- Soal Chapter 21Document5 pagesSoal Chapter 2121-096 Kharisma Theresia Adelina ButarButarNo ratings yet
- Multiple-Choice Test: 3 EnzymesDocument5 pagesMultiple-Choice Test: 3 EnzymesMuhammadNo ratings yet
- Aspen PlusDocument17 pagesAspen PlusNéia CostaNo ratings yet
- Practical Work Book: MY-201 Metallurgical Thermodynamics and KineticsDocument31 pagesPractical Work Book: MY-201 Metallurgical Thermodynamics and Kineticsk_banhawyNo ratings yet
- 1950, Von Bertalanffy, The Theory of Open Systems in Physics and BiologyDocument7 pages1950, Von Bertalanffy, The Theory of Open Systems in Physics and BiologyPancho MainländerNo ratings yet