Professional Documents
Culture Documents
Alcohol, Phenol - Ethers
Uploaded by
sarthakyedlawar04Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alcohol, Phenol - Ethers
Uploaded by
sarthakyedlawar04Copyright:
Available Formats
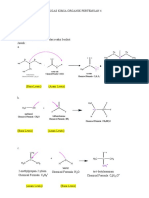
METHODS OF PREPARATION FROM HYDROBORATION
OF ALCOHOLS OXIDATION FROM DIAZONIUM SALTS METHODS OF PREPARATION
FROM CUMENE:
OF PHENOL
CH3 OH
CH3-CH=CH2 +(H-BH2)2 CH3-CH-CH2 N ≡ NCL OH CH3
From alkenes, by acid Catalysed Hydration: CH C–O–OH
H BH2 NaNO2 H2O CH3 O3 H+ + CH3COCH3
+ N2 + HCl DOW'S PROCESS:
+ HCl Warm
H CH3 H2O
C C
+ H2O C C (i) 623 K, 300 atmPhenol
CH3-CH=CH2
Chlorobenzene+ NaOH→
(ii ) HCl
NH2 Cumene Cumen
H OH CH3-CH=CH2
(CH3-CH2-CH2)3B (CH3-CH2-CH2)2BH Aniline
Benzene Hydroperoxide
Diazonium salt
H +
CH -CH-CH
eg. CH3-CH=CH2 + H2O CH3-CH=CH2
3 3
OH 3CH3-CH2-CH2-OH+B(OH)3
Colorless crystalline
Propan-l-ol FROM BENZENE SUPLHONIC ACID solid or Liquid
SO3H OH PHENOL PHYSICAL PROPERTIES
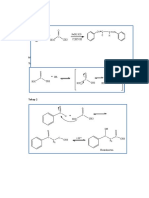
Reduction of Aldehyde, Ketone and
From Grignard reagent
Carboxylic Acid Oleum (i) NaOH Higher boiling point due
(ii) H+ to Hydrogen Bonding
pd
R − CHO + H 2 → R − CH 2 OH δ− +δ
C O + R + MgX C O Mg X CHEMICAL PROPERTIES
Halogeneation
NaBH
RCOR′
4 → R − CH − R
H2O OH OH
OH Electrophilic Aromatic Br Br
RCOOH
(i) LiAlH
(ii) H O
4 → R − CH − OH
2
2
Mg(OH)x + C OH
ALCOHOL, PHENOL substitution + 3Br2 + 3HBr
R
AND ETHER Br
Nitration
PHYSICAL PROPERTIES OH OH
ALCOHOLS Conc. NO2
NO2 NO2
HNO3
NO2
Soluble in water M.P. and B.P. ∝ Colorless with
ETHER 2,4,6-Trinitro-phenol
due to H-bonding Molecular mass M.P. characteristic Structure
and B.P. ∝ s smell R-OH Structure
where R = alkyl group R-O-R′
Reimer-Tiemann reaction: Treatement of
CHEMICAL PROPERTIES where R and R′ can be phenol with -
same or different alkyl CHCl3 + KOH O Na+
Reaction involving Acidity: Due to the presence of group CHCl2
OH
cleavage of -OH bond. polar-OH group. CHCl3 + Aq. NaOH
OH ONa R
R
R CH2OH > CH-OH > R C OH CLEAVAGE OF C-O BOND ELECTROPHILIC SUBSTITUTION
R Intermediate NaOH
+ NaOH + H 2O 1° alcohol
R 2° alcohol
R − O − R + HX
→ R − X + ROH
(I) HALOGENATION -
O Na
OCH3 CHO
R − O − R′ + HX
→ R − X + R′OH OCH3
CHO
OCH3 Br OH
Esterification H+
OH Br2 in Ethanoic Acid
H+ +
ROCOR′ + H 2 O
R − OH + R′COOH OR
H
+
ROCOR′ + R′COOH
R − OH + (R′CO) 2 O
CHEMICAL PROPERTIES + H-X + R-X Br Salicyaldehyde
Pyridine
R − OH + R′COCl
ROCOR′ + HCl For Tertiary Group:
CH3 CH3 (II) FRIEDEL-CRAFTS REACTION
Oxidation Phenol
OCH3 O
CH3 - C - OCH3 + HI → CH3OH + CH3 - C - I OH OH
Dehydration Reaction Oxidation OCH3 CH3 H3CO
AlCl3 Na2Cr2O7
CH3 CH3 + CH3Cl +
H O H2SO4
H+
C C
Heat
→ C + H 2O Oxidation [O]
H3C
R − CH 2 OH → R − C = O → R − C − OH O
Aldehyde Carboxylic Benzoquinone
H OH Acid PREPARATION OF ETHER
Victor Mayer Test
Primary Alcohol Secondary Alcohol Tertiary Alcohol
Williamson Synthesis By Dehydration of Alcohols
1) P+I2 1) P + I 2 1) P+ I 2
CHEMICAL TEST R − CH2 − OH → Red Colours – + 2CH 3 − CH 2 − OH →
2 4 H SO
C2 H5 − O − C2 H5
2) AgNO2 2) KOH → No Colours
AgNO NoReaction
R3C − OH →
: :
FOR ALCOHOL 2) AgNO R2CH − OH → Blue Colours R − X + R − O − Na
→ R − O − R + NaX 413 K
3) HNO
:
3) HNO , KOH 3) HNO , KOH
You might also like
- FROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENEDocument1 pageFROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENERonak kadamNo ratings yet
- ALCOHOL : AlcoholsDocument11 pagesALCOHOL : AlcoholsSamirNo ratings yet
- Organic TuteDocument1 pageOrganic TuteDimuthu SandaruwanNo ratings yet
- Allen Organic Quic RivisionDocument2 pagesAllen Organic Quic Rivisionsaisupreeth0913No ratings yet
- Allen Organic QUICK RevisionDocument2 pagesAllen Organic QUICK RevisionChetna Ahlawat100% (2)
- Phenols and Ethers NotesDocument9 pagesPhenols and Ethers NotesDhanaranjani BNo ratings yet
- Dia orDocument8 pagesDia orNaman MahawarNo ratings yet
- Alcohol Phenol Ether (1) 6Document9 pagesAlcohol Phenol Ether (1) 6sdnishacNo ratings yet
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Program Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsDocument2 pagesProgram Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsanis fazilaNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- Drug Abuse Ag8114en MKDocument56 pagesDrug Abuse Ag8114en MKA VegaNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- Aliphatic and Aromatic FlowchartDocument4 pagesAliphatic and Aromatic FlowchartapaperclipNo ratings yet
- Organic Chemistry Oxidation ReactionsDocument9 pagesOrganic Chemistry Oxidation Reactionsgamer boomerNo ratings yet
- Block Flow Diagram Prarancangan Pabrik Dimethyl Ether Dari Limbah Biomassa Perkebunan Kelapa Sawit DenganDocument2 pagesBlock Flow Diagram Prarancangan Pabrik Dimethyl Ether Dari Limbah Biomassa Perkebunan Kelapa Sawit DengandesniaNo ratings yet
- Important reactions of aromatic compounds summarizedDocument1 pageImportant reactions of aromatic compounds summarizedRoronoa ZoroNo ratings yet
- F H C B F F F Chemical Formula: CH BF ODocument3 pagesF H C B F F F Chemical Formula: CH BF OFadilla AzhariNo ratings yet
- carboxyl and amino questionsDocument6 pagescarboxyl and amino questionsatintasya18No ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- Exp 7 Preparation of AlkenesDocument14 pagesExp 7 Preparation of AlkenesGeorge PiliposyanNo ratings yet
- مقدمة في البحث (رنا الجهني)Document4 pagesمقدمة في البحث (رنا الجهني)Abdalmalek shamsanNo ratings yet
- 3CH CH CH CH + B H 3 (CH CH CH CH) B B (OH) +: (Basic Character)Document1 page3CH CH CH CH + B H 3 (CH CH CH CH) B B (OH) +: (Basic Character)SHADOW SNo ratings yet
- Aep 8Document6 pagesAep 8devkaushik0613No ratings yet
- Name Reaction TestDocument2 pagesName Reaction TestvikiasNo ratings yet
- Practic MapDocument1 pagePractic MapPrincessNo ratings yet
- Natural gas to methanol process block diagramDocument2 pagesNatural gas to methanol process block diagramAulia HasanahNo ratings yet
- 51LC S13 Elimination Background PDFDocument4 pages51LC S13 Elimination Background PDFButterlesstoastNo ratings yet
- S20 Ac Quim 10 Viellard EstebanDocument1 pageS20 Ac Quim 10 Viellard Estebanesteban viellardNo ratings yet
- Alcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024Document6 pagesAlcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024yash vardhanNo ratings yet
- Mekanisme RX DibenzalasetonDocument2 pagesMekanisme RX DibenzalasetonWulan safitriNo ratings yet
- Ethylene Glycol Mind MapsDocument1 pageEthylene Glycol Mind MapsHimanshu BhardwajNo ratings yet
- TM 2 Fundamentals of Organic Chemistry 7th Edition by John McMurryDocument15 pagesTM 2 Fundamentals of Organic Chemistry 7th Edition by John McMurrysukma AsaNo ratings yet
- CH - C-CH O OH OH: Clemmensen ReductionDocument4 pagesCH - C-CH O OH OH: Clemmensen ReductiondfghNo ratings yet
- Preparation and melting point of semicarbazone derivative of acetoneDocument2 pagesPreparation and melting point of semicarbazone derivative of acetonePawan SharmaNo ratings yet
- Alcohols, Phenols & Ether - AnswersDocument6 pagesAlcohols, Phenols & Ether - AnswersK. RupaNo ratings yet
- Cloxacillin QCDocument7 pagesCloxacillin QCSeLecToR ck LeeNo ratings yet
- Δ-Aminolevulinic Acid Synthase: Cooh CH CH C O Scoa + CH NH Cooh Succinyl Coash GlycineDocument10 pagesΔ-Aminolevulinic Acid Synthase: Cooh CH CH C O Scoa + CH NH Cooh Succinyl Coash Glycinevarsha CRNo ratings yet
- Name ReactionDocument7 pagesName ReactionSoumya KhatriNo ratings yet
- An Overview of Alkaloids: Their Classification, Extraction, Isolation, and Role in PlantsDocument49 pagesAn Overview of Alkaloids: Their Classification, Extraction, Isolation, and Role in PlantsmanishaNo ratings yet
- O N-NH: Wolf-Kishner ReductionDocument4 pagesO N-NH: Wolf-Kishner ReductiondfghNo ratings yet
- MIND MAP FOR PHENOL DERIVATIVESDocument1 pageMIND MAP FOR PHENOL DERIVATIVESPriyam PandaNo ratings yet
- Reaction SchemeDocument1 pageReaction SchemesakoakimNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Chapter 10 - Propylene Derivatives PDFDocument5 pagesChapter 10 - Propylene Derivatives PDFAlejandro Estrella GutiérrezNo ratings yet
- Chapter 6 BenzeneDocument9 pagesChapter 6 Benzenemeshal retteryNo ratings yet
- Peta Minda KimiaDocument36 pagesPeta Minda KimiaNATASHA 'ALIA BINTI ZULKIFLINo ratings yet
- Nanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnDocument12 pagesNanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnpNo ratings yet
- 05 Reductive AminationDocument2 pages05 Reductive AminationsubhasisknkNo ratings yet
- Sahd mdet Ras Lul! t cy0n HLi - CuBrtHBrDocument4 pagesSahd mdet Ras Lul! t cy0n HLi - CuBrtHBrSanskriti GuptaNo ratings yet
- Organic compounds structures and reactionsDocument2 pagesOrganic compounds structures and reactionsNicolas MartinezNo ratings yet
- Phenol : Mind MapsDocument1 pagePhenol : Mind Mapsbnnwdqwjz5No ratings yet
- 103 NOT OUT Organic ChemistryDocument1 page103 NOT OUT Organic ChemistryJeevan KumarNo ratings yet
- Parte 4Document1 pageParte 4Esteban Mauricio Viellard CorralesNo ratings yet
- CH CH CH CH I: BRCH CH CH CCH BR CH CHDocument24 pagesCH CH CH CH I: BRCH CH CH CCH BR CH CHSam TabujaraNo ratings yet
- Halogen DerivativeDocument6 pagesHalogen DerivativeSantanu DasNo ratings yet
- Chemical reactions and structuresDocument22 pagesChemical reactions and structuresStormy StudiosNo ratings yet
- Neutral pH Flow BatteryDocument1 pageNeutral pH Flow BatteryMonika Bartocha WróblewskaNo ratings yet
- CLS ENG 23 24 XII Che Target 1 Level 1 Chapter 2Document43 pagesCLS ENG 23 24 XII Che Target 1 Level 1 Chapter 2TirthNo ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular Structuresarthakyedlawar04No ratings yet
- Rotational MotionDocument1 pageRotational Motionsarthakyedlawar04No ratings yet
- P - Ch-28 - Communication SystemsDocument8 pagesP - Ch-28 - Communication Systemssarthakyedlawar04No ratings yet
- AIATS For First Step JEE (ADV) - Phase-3&4 Test-2A-P2 Code-H Sol 10-03-2024Document7 pagesAIATS For First Step JEE (ADV) - Phase-3&4 Test-2A-P2 Code-H Sol 10-03-2024sarthakyedlawar04No ratings yet
- A3 Classification of Elements and Periodicity in Properties MinDocument1 pageA3 Classification of Elements and Periodicity in Properties MinKarthikeyan LakshmananNo ratings yet
- Newton - S LawDocument1 pageNewton - S Lawsarthakyedlawar04No ratings yet
- Oscillations MindmapDocument1 pageOscillations Mindmapsarthakyedlawar04No ratings yet
- Environmental Chemistry.Document1 pageEnvironmental Chemistry.sarthakyedlawar04No ratings yet
- Chemistry in Everyday LifeDocument1 pageChemistry in Everyday Lifesarthakyedlawar04No ratings yet
- Thermodynamics-1 MindmapDocument1 pageThermodynamics-1 Mindmapsarthakyedlawar04No ratings yet
- Section - E: Subjective Type: G V T GT VDocument32 pagesSection - E: Subjective Type: G V T GT Vsarthakyedlawar04No ratings yet
- 6 General Principles and Processes of Isolation of Elements 1Document1 page6 General Principles and Processes of Isolation of Elements 1Raunak JayaswalNo ratings yet
- EquilibriumDocument1 pageEquilibriumsarthakyedlawar04No ratings yet
- ALLEN's DLP CBT/OFFLINE Exam Process and RequirementsDocument1 pageALLEN's DLP CBT/OFFLINE Exam Process and RequirementsVarun ChandelNo ratings yet
- SMA and Sofar Inverter PricelistDocument3 pagesSMA and Sofar Inverter PricelistYuvaraj JeyachandranNo ratings yet
- Vol06 Tab02Document387 pagesVol06 Tab02D2FNo ratings yet
- HTML Facebook CokDocument3 pagesHTML Facebook CokAldi SoNNo ratings yet
- Ohio EPA Sends Violation To Norwood Safety Service Director Joe GeersDocument5 pagesOhio EPA Sends Violation To Norwood Safety Service Director Joe GeersCincinnatiEnquirerNo ratings yet
- REPORT On Power Line Carrier CommunicationDocument28 pagesREPORT On Power Line Carrier CommunicationAshish Jain100% (4)
- 23-14683 HACCP Plan Review Checklist Rev. 09 01 17 PDFDocument4 pages23-14683 HACCP Plan Review Checklist Rev. 09 01 17 PDFIsna AndriantoNo ratings yet
- Sap Ehp 7 For Sap Erp 6Document9 pagesSap Ehp 7 For Sap Erp 6fiestamixNo ratings yet
- 710 CivDocument10 pages710 CivSamuel AguiarNo ratings yet
- Flowforming Mortar Cannon Barrels: Matthew FonteDocument2 pagesFlowforming Mortar Cannon Barrels: Matthew FonteAdnan ColoNo ratings yet
- Manual-Humdinger Concrete Vibrator 20151126 UKDocument20 pagesManual-Humdinger Concrete Vibrator 20151126 UKValentin NiculaeNo ratings yet
- Sewage System Design Spreadsheet-FinalDocument5 pagesSewage System Design Spreadsheet-FinalNwe OoNo ratings yet
- JMT Machine Tools (Press Brakes, Fiber Laser, Plasma Machines, Iron Worker, Shears, Angle Rolls,)Document78 pagesJMT Machine Tools (Press Brakes, Fiber Laser, Plasma Machines, Iron Worker, Shears, Angle Rolls,)Ermal HamzajNo ratings yet
- Typical Cable Laying Details For Direct Buried, Low Tension CablesDocument6 pagesTypical Cable Laying Details For Direct Buried, Low Tension CablesAbdul Muneer PalapraNo ratings yet
- Tumble Dryer Instruction BookletDocument72 pagesTumble Dryer Instruction BookletElla MariaNo ratings yet
- Brazo TB PDFDocument67 pagesBrazo TB PDFRimbertNo ratings yet
- 1 2 11 P Gliderdesign2Document2 pages1 2 11 P Gliderdesign2api-325611195No ratings yet
- Power and Eff WorksheetDocument6 pagesPower and Eff WorksheetYu ErinNo ratings yet
- Descriptive Statistics Using SPSS: Ex No: DateDocument5 pagesDescriptive Statistics Using SPSS: Ex No: Datepecmba11No ratings yet
- Anand Vihar Case StudyDocument4 pagesAnand Vihar Case StudyAnafNo ratings yet
- PROCESS COSTING PRODUCTION REPORTSDocument3 pagesPROCESS COSTING PRODUCTION REPORTSjozsefczNo ratings yet
- 2015 Ordinance BTech DtuDocument32 pages2015 Ordinance BTech DtuGaganpreetSinghNo ratings yet
- Mitsubishi Lifts BrochureDocument28 pagesMitsubishi Lifts Brochurenaveenarora298040No ratings yet
- Blackrock Methods of Delay AnalysisDocument36 pagesBlackrock Methods of Delay AnalysisAhmed MoubarkNo ratings yet
- Canon Irc3200 Parts CatalogDocument313 pagesCanon Irc3200 Parts CatalogStratis SiderisNo ratings yet
- Solar Wiring EbookDocument11 pagesSolar Wiring EbookLucian LazarutNo ratings yet
- Hammer Hlx5-Specs PDFDocument2 pagesHammer Hlx5-Specs PDFAlmaNo ratings yet
- Sizing a Flare Stack and Calculating Flame Distortion from Wind VelocityDocument49 pagesSizing a Flare Stack and Calculating Flame Distortion from Wind VelocityBayu AjipNo ratings yet
- Quality Test Bank 2Document16 pagesQuality Test Bank 2Behbehlynn100% (4)
- Parts Quotation for ENGKITDocument1 pageParts Quotation for ENGKITEslam MansourNo ratings yet
- Buried PipeDocument11 pagesBuried PipeAKKI KUMARNo ratings yet