Professional Documents
Culture Documents
Science Notes WITH CHEM
Uploaded by
AlfredCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Science Notes WITH CHEM
Uploaded by
AlfredCopyright:
Available Formats
Science Notes
Sunday, October 15, 2023 7:49 PM
Biology
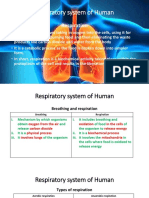
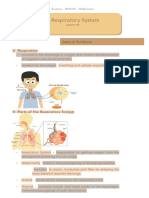
1.1 Respiration
• Respiration -A series of chemical reactions that happens inside every living cells in
the mitochondria. It releases all energy from the food and it is in all living things.
• Aerobic respiration – It is the process by cell in our body which converts oxygen and
glucose into energy, carbon dioxide and water.
• Anaerobic respiration - a chemical process in which energy is produced without the
use of oxygen.
Air Passage in order:
nose/mouth --> larynx (voice box) --> windpipe (trachea) --> bronchus --> bronchiole --> air
sacs ---> gas exchange
Parts of Respiration and its functions:
• Larynx (voice-box) - makes sounds, allows us to speak and supports as an air
passage.
• Trachea (windpipe) - carries air to the bronchus
• Bronchiole - carries air from the bronchus, deep into each lung
• Bronchus - delivers air to the lungs
• Air Sacs - where oxygen goes into the blood and carbon dioxide comes out
• Lungs - where oxygen gets into the body
• Ribs - protects the Lungs
Intercostal Muscle - contracts the ribs so the capacity of lungs get bigger.
• Diaphragm - the contracts and flattens and the chest cavity enlarges, which pulls air
into the lungs
Quick Notes Page 1
Ignore the eye, I got bored ok
How does air go into the body?
1. Air goes into the body through mouth (or) nose – connected with trachea
(windpipe) - around cartilage. The Cartilage keeps the windpipe open and
prevent collapsing – air moving in and out
2. Trachea divides into two bronchi (supported by cartilage)
3. A bronchus, each lung
4. A bronchus divides into bronchioles (several smaller tubes) allowing air to
reach deeper
5. Bronchioles end by air sacs (many tiny structures) where diffusion
happens (oxygen goes into blood and carries CO2 out)
Breathing and Respiration Difference:
→ Breathing - is the process of inhaling and exhaling of the gases between the cells
and the environment.
→ Respiration - is a chemical process that takes place in the cell. The air that we inhale
brings oxygen to the lungs and is carried by the blood to the cells, where it breaks
down glucose which creates energy.
1.2 Gas Exchange
Gas exchange is the process that happens inside the air sacs, oxygen from the air
Quick Notes Page 2
• Gas exchange is the process that happens inside the air sacs, oxygen from the air
goes into the blood and where the carbon dioxide from the blood goes back up into
the air.
• When oxygen gets into the blood, it dissolves, it goes into the red blood cells that
combines with hemoglobin, that is a red blood cell - that carries oxygen.
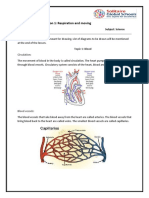
Air Sacs & Capillaries
• These holes are called air sacs. Another name for them is alveoli.
• There are also lots of very tiny blood vessels in the lungs, wrapped
around the air sacs. These blood vessels are capillaries.
Diffusion
• Diffusion is a process where oxygen particles move freely move through the thin
walled cells into the blood.
• In the diagram, the blood in a capillary on the left side has low oxygen and high
carbon dioxide. Meanwhile, air inside an air sac from outside the body has a lot of
oxygen and low in carbon dioxide. These gases exchange across the thin cell walls in
the alveolus which is where they transfer oxygen into the blood and take out carbon
dioxide.
Relationship between body mass the total area of air sacs
• The larger the body mass, the larger the total area of air sacs
• The larger the body mass of an organism, the more oxygen it will need because it
contains more cells that respires which are using up more oxygen.
• The total larger surface area needs more oxygen to diffuse into the body which helps
the respiring cells
Quick Notes Page 3
1.3 Breathing
• Breathing the process of moving air into and from the lungs to preform gas exchange,
to out carbon dioxide and bring in oxygen.
When you breathe in, these things happen:
• The intercostal muscles between the ribs contract (get shorter). This pulls the ribs
upwards and outwards.
• The diaphragm contract. This pulls the diaphragm downwards.
• They make up space inside the chest cavity. They increase the volume inside it.
• When the volume increases, the pressure inside the chest cavity and lungs
decreases.
• Air moves down through the trachea into the lungs, to fill the extra space.
When you breathe out, these things happen:
• The intercostal muscles between the ribs relax (return to normal size).
• This allow the ribs to drop down into their natural position.
• The muscles in the diaphragm relax. This allows the diaphragm to become its normal,
domed shape.
• These two movements make less space inside the chest cavity. They decrease the
volume inside it.
• When the volume decreases, the pressure increases. So, air is squeezed out of the
lungs.
1.4 Respiration
• Respiration is the process that the body uses to release energy from digested food
(glucose)
Facts About Glucose:
• All of our energy comes from the food that we eat.
• Carbohydrates are especially good for giving us energy.
• When we eat food containing carbohydrates, our digestive
• system breaks the carbohydrates down to a kind of sugar
• called glucose.
• The glucose goes into our blood.
• The blood delivers glucose to every cell in the body.
• The cells use the glucose to get the energy that they need.
Aerobic respiration formula
Glucose + Oxygen —› Carbon Dioxide + Water + Energy
[ Reactants ] [ Products ]
→ The air we breathe out contains less of the reactants (because they have been used
up by respiring cells) and, therefore, less oxygen. It contains more of the products
and, therefore, more carbon dioxide.
Heat Production
Quick Notes Page 4
• Chemical energy stored in the glucose is transferred to other substances, so the
cells can use it. In this process, some of the energy changes to heat energy. So the
respiring cells get warmer.
1.5 Blood
• Blood is red due to red blood cells
• The liquid part of the blood is called a blood plasma.
• The blood contains red blood cells
• The blood delivers oxygen and removes carbon dioxide, it moves around the body
inside the blood vessels. The heart pumps constantly to keep the blood moving.
Plasma
• Plasma is the liquid part of blood.
• Pale yellow liquid
• It is mostly water.
• The red and white blood cells are transported around the body in the blood plasma.
• Carbon dioxide is produced in every body cell by respiration.
• The carbon dioxide dissolves in blood plasma and is carried away from the cells.
• Transport red blood cells, white blood cells, dissolved glucose, dissolved carbon
dioxide.
How Cells deliver
• Every cell needs a good supply of glucose and oxygen
• The blood moves around the body inside blood vessels which keeps it pumping to keep
the blood moving.
Red Blood Cells White Blood Cells
They do not have nuclei or mitochondria They always have nuclei
Round dent in the middle, red spherical, colorless
Contains a pigment called hemoglobin which carries oxygen. They attach themselves with a chemical called antibodies to
kill pathogens
The oxygen combines with hemoglobin. Takes their cytoplasm and digests to kill them
It forms a very bright red compound called oxyhemoglobin It is called phagocytosis
Transports oxygen from the lungs to all cells in the body Destroy pathogens (bacteria, virus) that get in the body
As it has no nucleus it can have more space for haemoglobin The antibodies stick to the pathogen which kills them
directly sometimes.
It can travel through tiny capillary as is small Some white blood cells are larger
It won't use the carried oxygen as there is no mitochondria as They defend us from pathogens as they are harmful to us.
aerobic respiration happens inside the mitochondria
______________________________________________________________
Unit 4 - Ecosystem
4.1 The Sonoran Desert
Quick Notes Page 5
4.1 The Sonoran Desert
• Habitat - a place where an organism lives
• Ecosystem - a network of interactions between living and nonliving things which
contains many different habitats.
• Ecology - the study of ecosystems
• Predator - an animal that kills and eats other animals.
Food Chain
• It shows the transfer of energy within the ecosystem
• A food web consists of all the food chains in a single ecosystem
• The arrows represent energy in the form of chemical energy in food, passing from
one organism to another.
• A food chain consists of decomposer, producer, primary consumer, secondary
consumer, tertiary consumer and an apex predator
• The producers use energy from the Sun to make food by photosynthesis; this makes
energy available for all other organisms in the food web.
➢ Decomposer - an organism that breaks down dead organic material [fungus,
invertebrate]
➢ Producer - an organism that makes their down food by photosynthesis and has their
own source of energy where they don't have to rely to eat to gain energy [plants,
grass]
➢ Primary consumer - an organism, usually a herbivore, that eats the producer to gain
energy. [rabbit, grasshopper]
➢ Secondary consumer - an organism, usually a carnivore, that eats the primary
consumer to gain energy. [snake, fox]
➢ Tertiary consumer - an organism, usually a omnivore/carnivore, that eats the
secondary consumer [bear, wolf]
➢ Apex Predator - an organism (known as the top predator) is an organism at the top of
the food chain without natural predators of its own. [lion, tiger]
Quick Notes Page 6
4.2 Different Ecosystems
• Habitat - a place or area where an organism naturally resides in or where they live.
• Invasive species - Invasive species are animals or plants from another region of the
world that don't belong in their new environment.
Ecosystem
→ A network of interactions between living and nonliving things which contains many
different habitats.
→ They can be many types, some examples of them are mangrove forests, sea ice and
rice paddy field.
→ Not all of ecosystems are natural like the rice paddy field, as they are grown by
people and it serves as a natural habitat for fish, birds and algae.
______________________________________________________________
Chemistry
2.1 Dissolving
3 states of matter:
Solid
Quick Notes Page 7
➢ Solid
➢ Liquid
➢ Gas
• Dissolve - a solid gradually disappearing in a liquid
• Solute - a substance that disappears/dissolve [salt, sugar]
• Solvent - the substance the solute dissolves in [water]
• Solution - a mixture of solute and solvent [salty water]
• Opaque - unable to see through it because it is blocking light
• Transparent - able to see it because light passes through
• Translucent - not able to see it clearly because only some light passes through
• Element - a single material like gold
• Compound - two materials like sedimentary rock, hard to separate as atoms of
different elements are chemically combined.
• Mixture - a compound and element mixed by two or more materials.
2.2 Solutions and Solubility
• Concentrated Solution - a solution in which a large amount of solute is dissolved
• Dilute - a solution in which a small amount of solute is dissolved
• Soluble - substance that will dissolve in a given solvent
• Insoluble - substance that will not dissolve in a given solvent
• Saturated solution - a solution in which no more solution can be dissolved
Comparing solubility
• To compare the solubility of different solutes you must measure how much solute will
dissolve in a known amount of solvent
Temperature and Solubility
• Most solute will dissolve more quickly and easily in hot water than cold water
2.3 Planning a solubility investigation
• Variable - things can be changed in an investigation
• Independent variable - the variable that is changed in an investigation [Temperature]
• Dependent variable - the variable that changed in an investigation due to the
independent variable, can be measured [Number of Spatulas]
• Control variable - things that are kept the same in the investigation so the test is
fair [Volume of water]
• Range - the difference between the highest and lowest values
• Interval - the size of the change of value of the independent variable
2.4 Chromatography
• Paper chromatography - a method for separating mixtures of dissolved chemicals
using a special paper
Quick Notes Page 8
using a special paper
• Chromatogram - the resulting separation of substance's after carrying out a
chromatogram.
• Doing a test again results in more accurate results.
______________________________________________________________
Unit 5 Materials and the structure
Unit 5.1 The structure of the atom
• Atoms - tiny particles of matter
• Deflected - causes something to change directions to turn aside from a straight
course
• Electrical charge - physical property of matter that causes force when placed a
electromagnetic field
• Electrons 0 negatively charged particles, no mass
• Protons - positively charged particles [ Protons and
Electrons are the Same size and mass]
• Neutrons - negatively charged particles
• Neutrons - neutral charged particles
• Subatomic particles - particles that are found inside an atom
Element Key
It shows an element's chemical symbol, atomic number and mass
- Atomic Number = Proton
Quick Notes Page 9
JJ Thompson
• British scientist
• Discovered electrons in 1890's
• In his model, electrons are scattered throughout the structure
• Plum pudding model (fruit cake)
Ernest Rutherford
• Scientist from New Zealand
• Discovered protons in 1909
• Discovered nucleus in 1911
• Gold foil experiment
• His experiment fired fast moving particles, at a very thin gold foil, most particles
passed through the foil, only few particles (1 in 1800) got deflected (direction of it
was changed)
James Chadwick
• Scientist from New Zealand with Ernest Rutherford
• Discovered neutrons in 1932
Quick Notes Page 10
•
5.2 Purity
• Purity is how pure a substance is
Purity of Silver
• Examples of silver objects are silverware and rings
• To calculate the purity you divide the parts out of 1000 parts
• 925/100 x 100 = 92.5% purity
Purity of Carbon
• Examples of carbon are charcoal, coal and diamond
• Diamonds are made from carbon
• Pure diamonds are colorless and translucent
• If mixed with nitrogen it will be yellow
• If mixed with boron, it will be blue
• Rarest diamond is green, it is formed when atom per 100 of carbon atoms are
replaced by nitrogen, nickel or hydrogen
Purity of Gold
• Purity of gold is 24K (24 Carat)
• It changes from coppery to yellow-gold color depending on its purity
• To calculate you have to calculate, you divide the parts out of 24 parts
• 18/24 x 100 = 75%
Quick Notes Page 11
You might also like
- Lung Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandLung Diseases, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Respiration: How Cells Convert EnergyDocument45 pagesRespiration: How Cells Convert EnergyMaleeha HumayunNo ratings yet
- 2023 Science Progression Test Study GuideDocument29 pages2023 Science Progression Test Study Guideahaja ahajoNo ratings yet
- Respiration: BiologyDocument45 pagesRespiration: BiologySyed HalawiNo ratings yet
- Respiratory System: The Organs That Help To Get Oxygen From The Air Into Your Blood, and To Get Rid of Carbon DioxideDocument22 pagesRespiratory System: The Organs That Help To Get Oxygen From The Air Into Your Blood, and To Get Rid of Carbon DioxideTravel UnlimitedNo ratings yet
- IGCSE Biology: Respiration and Gas ExchangeDocument29 pagesIGCSE Biology: Respiration and Gas Exchangemiriam odonkorNo ratings yet
- Chapter 1Document17 pagesChapter 1wallacec1017No ratings yet
- 7th Grade Biology Notes on Respiration and Gas ExchangeDocument6 pages7th Grade Biology Notes on Respiration and Gas ExchangeSahithi PNo ratings yet
- 6454f627e4b0ad3aaf22e06b OriginalDocument13 pages6454f627e4b0ad3aaf22e06b OriginalSuprit JainNo ratings yet
- Respiration BiologyDocument32 pagesRespiration BiologyMuhammad KhanNo ratings yet
- S2 - Bio - RevisionNotes 1.1 To 1.4 - PreMidDocument7 pagesS2 - Bio - RevisionNotes 1.1 To 1.4 - PreMidKaung ThihaNo ratings yet
- Unit 1.1. Cardio-Respiratory SystemDocument43 pagesUnit 1.1. Cardio-Respiratory SystemCarmsNo ratings yet
- WWW Studyrankers Com 2017 07 Notes of CH 6 Life Process Part II Class 10th HTMLDocument15 pagesWWW Studyrankers Com 2017 07 Notes of CH 6 Life Process Part II Class 10th HTMLbaapmatsikha5No ratings yet
- IGCSE Biology NotesDocument9 pagesIGCSE Biology NotesJEFF0% (1)
- Gaseous Exchange in The Mammalian Lungs: by Vytautas KontrimasDocument10 pagesGaseous Exchange in The Mammalian Lungs: by Vytautas KontrimascluendoNo ratings yet
- Chapter 1 NotesDocument12 pagesChapter 1 NotesLim Zhi EnNo ratings yet
- Unit 1 RespirationDocument18 pagesUnit 1 Respiration4w7b9kkyp8No ratings yet
- Respiratory SystemDocument19 pagesRespiratory SystemMotiourNo ratings yet
- The Respiratory System and RespirationDocument7 pagesThe Respiratory System and RespirationAntonateNo ratings yet
- What Is Respiration?Document8 pagesWhat Is Respiration?Waleed Bin KhalidNo ratings yet
- Life Process RespirationDocument51 pagesLife Process RespirationJatin Kalra 10 DNo ratings yet
- Respiration and Gas ExchangeDocument48 pagesRespiration and Gas ExchangeIbrahim NOORZADNo ratings yet
- Respiration and Gas ExchangeDocument2 pagesRespiration and Gas ExchangeAnime Player ccNo ratings yet
- Human Respiratory System: Gas Exchange and the LungsDocument21 pagesHuman Respiratory System: Gas Exchange and the LungsEnmuskNo ratings yet
- Chapter 11 - Respiration and Gas ExchangeDocument64 pagesChapter 11 - Respiration and Gas ExchangeKareem DmourNo ratings yet
- Energy Producing & Distributing SystemsDocument40 pagesEnergy Producing & Distributing SystemsZerille Anne InsonNo ratings yet
- Summary Unit 3. Systems of The Nutrition FunctionDocument29 pagesSummary Unit 3. Systems of The Nutrition FunctionwendyNo ratings yet
- RESPIRATIONDocument11 pagesRESPIRATIONCREATIVING TAMILANNo ratings yet
- Class 7 RespirationDocument5 pagesClass 7 RespirationGopi SNo ratings yet
- Respiratory System: Gas ExchangeDocument30 pagesRespiratory System: Gas ExchangeDawn Regile MorilloNo ratings yet
- Circulatory System & Respiration HODocument7 pagesCirculatory System & Respiration HODina Ayudan CortezNo ratings yet
- Respiratory System in Human BeingsDocument11 pagesRespiratory System in Human BeingsSarada KasyapNo ratings yet
- Ch-11 and Ch-12 Gas-Exchange in Humans and RespirationDocument12 pagesCh-11 and Ch-12 Gas-Exchange in Humans and Respirationdaveymilan36No ratings yet
- Respiration Grade 10 Biology - StudentDocument65 pagesRespiration Grade 10 Biology - StudentAsawni McDowellNo ratings yet
- Plants Make Own Food Through PhotosynthesisDocument15 pagesPlants Make Own Food Through PhotosynthesispranayaNo ratings yet
- Form 3 Science Chapter 1Document25 pagesForm 3 Science Chapter 1Mukilan MurugasanNo ratings yet
- Aerobic vs Anaerobic RespirationDocument5 pagesAerobic vs Anaerobic Respirationsriniram_2002No ratings yet
- Respiratory SystemDocument14 pagesRespiratory SystemTedi FetoNo ratings yet
- Chapter 11 Bio NotesDocument4 pagesChapter 11 Bio NotesArabella ShepherdNo ratings yet
- Respiration 8c RevisionDocument4 pagesRespiration 8c RevisionJelle MustefaNo ratings yet
- Respiratory System of HumanDocument35 pagesRespiratory System of HumanBlister CountNo ratings yet
- Breathing and Gaseous Exchnage by Sherrie-Ann Wilson-BrownDocument85 pagesBreathing and Gaseous Exchnage by Sherrie-Ann Wilson-BrownNayoka EnnisNo ratings yet
- Animal Organ SystemDocument139 pagesAnimal Organ SystemMilyn Mae PalganNo ratings yet
- Lesson 1 Respiration and MovingDocument8 pagesLesson 1 Respiration and MovingCHENNAIAH GARI SAI BABUNo ratings yet
- Respiration NotesDocument7 pagesRespiration NotesTamisha JacobsNo ratings yet
- Respiration, Transport and ExcretionDocument14 pagesRespiration, Transport and ExcretionAmjad KhanNo ratings yet
- Biology Semester 1 FT Grade 8Document10 pagesBiology Semester 1 FT Grade 8SMii7YNo ratings yet
- Science 9: 1St QuarterDocument42 pagesScience 9: 1St QuarterCharmel Joy TeopeNo ratings yet
- RespirationDocument35 pagesRespirationnabeeha malhiNo ratings yet
- Diving PhysiologyDocument334 pagesDiving PhysiologyemilidiverNo ratings yet
- Chapter 1 Part B - Respiration, Breathing and Circulation NotesDocument15 pagesChapter 1 Part B - Respiration, Breathing and Circulation NotesHailey CaruanaNo ratings yet
- Digestive and Respiratory SystemDocument48 pagesDigestive and Respiratory Systemkenneth vergaraNo ratings yet
- Human Respiratory SystemDocument11 pagesHuman Respiratory SystemTania Ferdous RipaNo ratings yet
- Biology Fluid RegulationDocument10 pagesBiology Fluid RegulationArcely EchaniqueNo ratings yet
- Gas Exchange in AnimalsDocument20 pagesGas Exchange in AnimalsMaria Theresa HerreroNo ratings yet
- Respiratory System: Parts & FuntionsDocument16 pagesRespiratory System: Parts & FuntionsAlthaea LiceraNo ratings yet
- Human Respiratory SystemDocument32 pagesHuman Respiratory SystemOyrik Mukhopadhyay100% (1)
- Biology - Unit 3: Dissolved SubstancesDocument11 pagesBiology - Unit 3: Dissolved SubstancesTahirNo ratings yet
- Respiratory SystemDocument28 pagesRespiratory SystemVanezaKateYeclaRetinioNo ratings yet
- Respiration NotesDocument7 pagesRespiration NotesKrishna SharmaNo ratings yet
- History_practice_ansDocument2 pagesHistory_practice_ansAlfredNo ratings yet
- History NotesDocument12 pagesHistory NotesAlfredNo ratings yet
- AfricaDocument13 pagesAfricaAlfredNo ratings yet
- The Amritsar MassacreDocument3 pagesThe Amritsar MassacreAlfredNo ratings yet
- GP Debate YesDocument6 pagesGP Debate YesAlfredNo ratings yet
- GP Debate YesDocument6 pagesGP Debate YesAlfredNo ratings yet
- Substances Harmful To The Respiratory SystemDocument25 pagesSubstances Harmful To The Respiratory SystemKar Wai67% (3)
- When Formulating A Hypothesis, It Must Be: Form 4 Biology Question BankDocument28 pagesWhen Formulating A Hypothesis, It Must Be: Form 4 Biology Question BankAnas IsmailNo ratings yet
- Drugs Acting On The Respiratory SystemDocument13 pagesDrugs Acting On The Respiratory SystemNathalie kate petallarNo ratings yet
- Respiratory Therapy FormulasDocument3 pagesRespiratory Therapy Formulasrtman50% (2)
- Acitivty Sheets Q1W1 W2Document8 pagesAcitivty Sheets Q1W1 W2Christian BejarinNo ratings yet
- CX Xray PresentationDocument75 pagesCX Xray PresentationPrince RichardNo ratings yet
- Snake AnatomyDocument7 pagesSnake AnatomyAdrin Ma'rufNo ratings yet
- Seminar On Bronchial Asthma NewDocument42 pagesSeminar On Bronchial Asthma NewShabna SameerNo ratings yet
- Unit:11 (Burn Injury)Document6 pagesUnit:11 (Burn Injury)Surkhali BipanaNo ratings yet
- Mechanism Of Swallowing: Stages And CoordinationDocument6 pagesMechanism Of Swallowing: Stages And CoordinationLittle MummutNo ratings yet
- Res 216 QaDocument118 pagesRes 216 QaDoaa Zakaria AliNo ratings yet
- Bronchial Asthma (Case Study)Document12 pagesBronchial Asthma (Case Study)Adriane Coma100% (1)
- Agricultural Science For Secondary School Book 3Document319 pagesAgricultural Science For Secondary School Book 3Geofrey83% (6)
- sbl1023 Lab 7 Human PhysiologyDocument8 pagessbl1023 Lab 7 Human Physiologyapi-385146128No ratings yet
- TOEFL Ibt Çıkmış Soruları Set 2 Kitap 2Document69 pagesTOEFL Ibt Çıkmış Soruları Set 2 Kitap 2online toeflNo ratings yet
- End Blok Respi SoalDocument6 pagesEnd Blok Respi Soalanz_4191No ratings yet
- Matias - PEX 07 03Document7 pagesMatias - PEX 07 03Mae MatiasNo ratings yet
- Airway ManagementDocument38 pagesAirway ManagementyafetNo ratings yet
- Speech Organs PracticeDocument2 pagesSpeech Organs PracticeEsther de RiviaNo ratings yet
- Pulmonary Pathology, An Atlas andDocument1,604 pagesPulmonary Pathology, An Atlas andDANIEL IZE RONCHINo ratings yet
- Assessmen T Nursing Diagnosis Nursing Goal Intervent Ions Rational E Outcome CriteriaDocument2 pagesAssessmen T Nursing Diagnosis Nursing Goal Intervent Ions Rational E Outcome CriteriaMiguelito Galagar GultianoNo ratings yet
- Breathing and Exchange of GasesDocument54 pagesBreathing and Exchange of GasesShuryans MishraNo ratings yet
- Parts of Resperatory SystemDocument5 pagesParts of Resperatory SystemPeysbukan Cyber CafeNo ratings yet
- Miller Airway ManagementDocument65 pagesMiller Airway ManagementBenediktus BayuNo ratings yet
- Manual Drager MedicalDocument8 pagesManual Drager MedicalPaolaRubianoNo ratings yet
- Human Diseases Case Study 19CDocument3 pagesHuman Diseases Case Study 19Cairickann50% (2)
- LIFE PROCESSES - Question BankDocument15 pagesLIFE PROCESSES - Question BankMISHKA KHANDELWALNo ratings yet
- Surfactant Replacement TherapyDocument42 pagesSurfactant Replacement TherapyPayas JoshiNo ratings yet
- LOWTEM Smart 150D Service Manual (en-LTS150DSE - Rev02) Apr 2018Document44 pagesLOWTEM Smart 150D Service Manual (en-LTS150DSE - Rev02) Apr 2018Adrian Del Castillo Velasco100% (1)
- Pulmo Management Week 1Document160 pagesPulmo Management Week 1Dharlyn MungcalNo ratings yet
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Chemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)From EverandChemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)No ratings yet
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)