Professional Documents
Culture Documents
Ch05 Liquid - Solid Separation - Leaching
Uploaded by
SyShobanaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ch05 Liquid - Solid Separation - Leaching
Uploaded by
SyShobanaCopyright:
Available Formats
+
BKF3463
UNIT OPERATION 1
Dr. Syed Mohd Saufi
2012/2013-I
1
Chapter 5
Part 2:
Liquid-Solid Separation
(Leaching)
+
Introduction
Separation of components/solute from solid by contacting with
a liquid phase
The two phases are in intimate contact and the solute(s) can
diffuse from the solid to the liquid phase, resulting the
separation of components originally in the solid called as
liquid-solid leaching or leaching
When an undesirable component is removed from a solid with
water, the process is called washing
Example leaching process
leaching of sugar from sugar beets
vegetable oil leaching from peanuts/soybeans/sunflower/etc using
solvent hexane/acetone/ether/etc
metal processing industries copper salts leached from ground
ores by sulfuric acid or ammoniacal solutions
Dr SMS 2012/2013
2
+
Preparation of Solids for Leaching
This depends on the proportion of the soluble constituent
present, its distribution throughout the original solid, the nature
of the solid, and the original particle size.
If the soluble material is surrounded by a matrix of insoluble
matter, the solvent must diffuse inside to contact and dissolve
the soluble material and then diffuse out.
This is common in leaching metal salts from mineral ores. In
these cases crushing and grinding of the ores is used to
increase the rate of leaching since the solution portions are
made more accessible to the solvent.
Biological materials are cellular in structure and the soluble
constituents are inside the cells. Because the cell walls provide
another resistance to diffusion, the rate of leaching may be
slow.
In this case the biological materials are cut into thin wedge-
shaped slices to reduce the diffusion distance of solvent.
Dr SMS 2012/2013
3
+
Rates of Leaching
Generally there are five rate steps in the leaching process:
1. The solvent is transferred from the bulk solution to the surface of
the solid.
2. The solvent penetrates or diffuses into the solid (intraparticle
diffusion).
3. The solute dissolves from the solid into the solvent.
4. The solute diffuses through the mixture to the surface of the solid
(intraparticle diffusion).
5. The solute is transferred to the bulk solution.
Step 1 is usually fast. The controlling rate process is generally
the intraparticle diffusion or the dissolving step.
Dr SMS 2012/2013
4
+
Type of Equipment for Leaching
Fixed bed leaching
Dr SMS 2012/2013
5
+
Type of Equipment for Leaching
Moving bed leaching
Dr SMS 2012/2013
6
+
Type of Equipment for Leaching
Agitated solid leaching
Dr SMS 2012/2013
7
+
Equilibrium Relations in Leaching
Assumption
Sufficient solvent present so that all the solute in the entering solid
can be dissolved into the liquid, equilibrium is reached when the
solute is dissolved. Hence, all the solute is completely dissolved in
the first stage.
The solid is insoluble,
No adsorption will happen for the solute in the solid, meaning that
the solution in the liquid phase leaving a stage is the same as the
solution remaining with the solid matrix in the settled slurry leaving
the same stage.
The settled solid leaving a stage always contains some liquid. This
solid-liquid stream is called the underflow or slurry stream.
The liquid is called the overflow stream.
The concentration of oil or solute in the overflow stream is equal to
that in the liquid solution accompanying the slurry or underflow
stream. Hence, on an xy plot the equilibrium line is on the 45o line.
Dr SMS 2012/2013
8
+
Equilibrium Diagrams For Leaching
The concentration of inert or
insoluble solid B in the solution
mixture or the slurry mixture is
defined as N
For the overflow, N = 0, for the
underflow, N depends on the
solute concentration in the liquid.
The compositions of solute A in
the liquid as weight fractions are
For the entering solid feed to be
leached, N is kg inert solid/kg
solute A and y
A
= 1.0.
For pure entering solvent N = 0
and x
A
= 0.
Dr SMS 2012/2013
9
+
Single-Stage Leaching
Material balance: V is kg/h of overflow solution with composition xA and L is the
kg/h of liquid in the slurry solution with composition yA based on a given flow
rate B kg/h of dry solute-free solid.
A balance on C is not needed since x
A
+ x
C
= 1 and y
A
+ y
C
= 1
M is the total flow rate in kg (A+C)/h, x
AM
and N
M
are the coordinates of this
point M.
If L
0
entering is the fresh solid feed to be leached with no solvent C present, it
would be located above the N versus y line in the below figure.
Dr SMS 2012/2013
10
+
Example 12.9-1
In a Single-Stage Leaching of soybean oil from flaked soybeans with
hexane, 100 kg of soybeans containing 20 wt % oil is leached with 100
kg of fresh hexane solvent. The value of N for the slurry underflow is
constant at 1.5 kg insoluble solid/kg solution retained. Calculate the
amounts and compositions of the overflow V
1
and the underflow L
1
leaving the stage.
Dr SMS 2012/2013
11
+
Countercurrent Multistage Leaching
The number of stages are numbered in the direction of the solids/underflow
stream
Note that the composition of the V phase is denoted by x and the composition
of the L phase by y, which is the reverse of that for liquid-liquid extraction
Material balance and operating line
This can be plotted on an xy plot passes through the terminal points (x1, y0)
and (xn+1, yn).
Dr SMS 2012/2013
12
+
Countercurrent Multistage Leaching
Dr SMS 2012/2013
13
If the viscosity and density of the solution change with the
solute (A) concentration, the liquid retained in the solid
underflow will change and so will the overflow and the slope of
the operating line vary. This is called variable underflow.
If the amount of solution Ln retained by the solid is constant
and independent of concentration, then we will have constant
underflow.
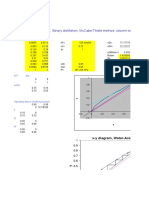
+ Countercurrent Multistage Leaching:
Variable Underflow
Dr SMS 2012/2013
14
Usually the flows and compositions of L
0
and
V
N+1
are known and the desired exit
concentration y
A
, N is set. Then the
coordinates N
M
and x
AM
can be determined.
This point can also be
located graphically as
the intersection of lines
L
0
V
1
and L
N
V
N+1
.
To graphically determine the number of stages, we start at L0
and draw a line L0 to locate V1. Then an equilibrium tie line
through V1 locates L1. Line L1 is drawn to give V2. A tie line
from V2 gives L2. This is continued until the desired LN is
reached.
+ Countercurrent Multistage Leaching:
Constant Underflow
Dr SMS 2012/2013
15
In this case the liquid L
n
retained in the underflow solids is
constant from stage to stage. This means that a plot of N versus y
A
is a horizontal line and N is constant.
For constant underflow, it is possible to use the McCabe and
Thiele method on the xy diagram
The operating line is now a straight line and the equilibrium line is
usually linear (y
A
= x
A
)
However, special treatment must be given for the first stage,
because L
0
is generally not equal to L
n
, since it contains little or
no solvent.
A separate material balance must be made on stage 1 to obtain L
1
and V
2
. Then the straight operating line can be used and the
McCabe Thiele method used to step off the number of stages
The calculation procedure discussed in the previous section for
variable underflow can still be used for constant underflow by
simply using a horizontal line of N versus y
A
and stepping off the
stages with the point.
+
Example 12.10-1
Dr SMS 2012/2013
16
+
Example 12.10-1
Dr SMS 2012/2013
17
+
Thank You
Dr SMS 2012/2013
18
You might also like
- 6 High Efficiency Boiler Technology Sugar Industry Suwat enDocument29 pages6 High Efficiency Boiler Technology Sugar Industry Suwat enctomeyNo ratings yet
- Wheat Milling and Baking Technology Foodkida-1Document8 pagesWheat Milling and Baking Technology Foodkida-1Sumit KumarNo ratings yet
- Unit 11 Softy and Novelties - Definition, Composition, Legal Standards and Method of ManufactureDocument15 pagesUnit 11 Softy and Novelties - Definition, Composition, Legal Standards and Method of ManufactureRonak RawatNo ratings yet
- Sugar Technology: Technologie GMBHDocument8 pagesSugar Technology: Technologie GMBHBala MuruganNo ratings yet
- SOP - Startup Shutdown and Operation of Coal MillDocument2 pagesSOP - Startup Shutdown and Operation of Coal MillJCSNo ratings yet
- Engineering College Sugar Industry Waste Water ReportDocument20 pagesEngineering College Sugar Industry Waste Water ReportAditi SharmaNo ratings yet
- The Use of Scrap Tires in Rotary Cement KilnsDocument9 pagesThe Use of Scrap Tires in Rotary Cement KilnsrpazbNo ratings yet
- Sugar Processing StepsDocument17 pagesSugar Processing StepsTehreem_2000No ratings yet
- Heat and Mass BalanceDocument16 pagesHeat and Mass BalanceAndy TpNo ratings yet
- Portland CementDocument58 pagesPortland CementNani DeskaaNo ratings yet
- Commercial Pharmaceutical FormulationsDocument38 pagesCommercial Pharmaceutical FormulationsSam WanneNo ratings yet
- Dry ProcessDocument4 pagesDry ProcessFahamidur Rahman ShawonNo ratings yet
- Filtration Techniques for Separating Liquids and SolidsDocument18 pagesFiltration Techniques for Separating Liquids and SolidsPooja Choudhary100% (1)
- Isomerization of Glucose To FructoseDocument4 pagesIsomerization of Glucose To FructoseOoi Ah GuanNo ratings yet
- Taye Teachingh PractiseDocument9 pagesTaye Teachingh Practisetsegaye atnafuNo ratings yet
- Waste Water Treatment in Dairy IndustriesDocument14 pagesWaste Water Treatment in Dairy Industries19 CH 056 Vaishali Vivek100% (1)
- Map of a Limestone Quarry and Cement Plant Key AreasDocument1 pageMap of a Limestone Quarry and Cement Plant Key Areasasif_rahman06No ratings yet
- Roller Press (High Pressure Grinding Rolls) : V Naga KumarDocument24 pagesRoller Press (High Pressure Grinding Rolls) : V Naga KumarApril ReynoldsNo ratings yet
- Latest Technology in Cereals ProductionDocument5 pagesLatest Technology in Cereals ProductionNadherah MohamadNo ratings yet
- Diffusion Vs MillingDocument4 pagesDiffusion Vs MillingcbqucbquNo ratings yet
- Pelleting ProcessDocument59 pagesPelleting ProcessMinh PhamNo ratings yet
- Yeast ProductionDocument7 pagesYeast Productionboonsom100% (1)
- Oxygen TransferDocument37 pagesOxygen TransferKaycee ChirendaNo ratings yet
- Inorganic Chemical Industries: Chlor-alkali, Ammonia, Sulphuric Acid & Fertilizer ProcessesDocument31 pagesInorganic Chemical Industries: Chlor-alkali, Ammonia, Sulphuric Acid & Fertilizer ProcessesSophia WambuiNo ratings yet
- Description of Process - Nbsm-1Document10 pagesDescription of Process - Nbsm-1avisheklochunNo ratings yet
- Sugar Cane JuiceDocument5 pagesSugar Cane Juices.sabapathyNo ratings yet
- Material Balance in MillDocument6 pagesMaterial Balance in MillNikhilNo ratings yet
- SMP Skim Milk Powder StandardsDocument2 pagesSMP Skim Milk Powder StandardsShubham DubeyNo ratings yet
- Finamul 97 SSL: Product Data SheetDocument1 pageFinamul 97 SSL: Product Data SheetToni AcoskiNo ratings yet
- Carbonless Refining of Starch Hydrolyzates with Macronet AdsorbentsDocument14 pagesCarbonless Refining of Starch Hydrolyzates with Macronet AdsorbentsherdianpebiNo ratings yet
- Aeration of Cereal Dough by YeastDocument13 pagesAeration of Cereal Dough by Yeastkolita kamal100% (1)
- Cause of Materials Accumulation in CycloneDocument3 pagesCause of Materials Accumulation in CyclonekidcatNo ratings yet
- Managing Solid Waste EffectivelyDocument11 pagesManaging Solid Waste Effectivelydaabgchi100% (1)
- Corn Steep Liquor in Microbiology PDFDocument15 pagesCorn Steep Liquor in Microbiology PDFNasser KemmouNo ratings yet
- Sachit Liquid Glucose Bsi FinalDocument50 pagesSachit Liquid Glucose Bsi FinalSachit GambhirNo ratings yet
- Manual RF Baghouse CollectorDocument24 pagesManual RF Baghouse Collectorheroj83100% (1)
- Cereal Technology NotesDocument45 pagesCereal Technology Notesgrace mwenjeNo ratings yet
- Sugar Production ProcessDocument2 pagesSugar Production ProcessedmarkNo ratings yet
- Ball Mill Machine Guide - Less than 40 CharactersDocument2 pagesBall Mill Machine Guide - Less than 40 Characterspadma26327No ratings yet
- Preventing Unsoundness in High-MgO CementsDocument19 pagesPreventing Unsoundness in High-MgO CementsYuniar Luthfia ListyadeviNo ratings yet
- Flour & Product Quality Testing EquipmentsDocument31 pagesFlour & Product Quality Testing EquipmentsrishabhwillkillyouNo ratings yet
- Pyroprocessing: A Concise Guide to the High-Temperature Conversion of Raw Materials into ClinkerDocument27 pagesPyroprocessing: A Concise Guide to the High-Temperature Conversion of Raw Materials into ClinkerTitan Titanovsky KoraagNo ratings yet
- Paper Darab Cement Kiln SealDocument11 pagesPaper Darab Cement Kiln Sealomid1302No ratings yet
- Cement GrindingDocument19 pagesCement GrindingCao Ngoc AnhNo ratings yet
- Cement Industry: Handy ManualDocument54 pagesCement Industry: Handy Manualcostea0028No ratings yet
- Operacion 44Document44 pagesOperacion 44Nelly Isabel Narvaez PachecoNo ratings yet
- Chapter 1Document56 pagesChapter 1Surbhi JainNo ratings yet
- 1 Homogenization PDFDocument9 pages1 Homogenization PDFLê Hoàng Anh TuấnNo ratings yet
- Analyzing Fiber in Raspberries Using Proximate AnalysisDocument5 pagesAnalyzing Fiber in Raspberries Using Proximate AnalysisKaye Danielle HilomenNo ratings yet
- 01 - EX Kit - FMF - Electronic BookletDocument36 pages01 - EX Kit - FMF - Electronic BookletGerman AnayaNo ratings yet
- Caustic Soda From Natural Trona 2014Document5 pagesCaustic Soda From Natural Trona 2014MauRmzNo ratings yet
- Flow Process Cement Plant Quality ControlDocument10 pagesFlow Process Cement Plant Quality ControlAgung BinantoroNo ratings yet
- Collaboration Cuts Costs and Increases Capacity by 35%: CASE: India Cements Limited, ChilamkurDocument4 pagesCollaboration Cuts Costs and Increases Capacity by 35%: CASE: India Cements Limited, ChilamkurjmpbarrosNo ratings yet
- Pharmaceutical Granulation Equipment GuideDocument19 pagesPharmaceutical Granulation Equipment GuideJoslin RozNo ratings yet
- 1Document5 pages1Dee HsNo ratings yet
- Chopin Tribune 1 Uk (Ene.1996)Document4 pagesChopin Tribune 1 Uk (Ene.1996)MALELA70No ratings yet
- Modul EkstraksiDocument11 pagesModul EkstraksiBayu Octavian PrasetyaNo ratings yet
- 221 07 PDFDocument11 pages221 07 PDFFarah Talib Al-sudaniNo ratings yet
- Unit Operations 2 Laboratory Experiment 8 Batch Leaching of Nacl-Sand Mixture With Water As SolventDocument13 pagesUnit Operations 2 Laboratory Experiment 8 Batch Leaching of Nacl-Sand Mixture With Water As SolventMeredith VillareteNo ratings yet
- Ideal LeachingDocument15 pagesIdeal LeachingSata AjjamNo ratings yet
- Glycerol MethodsDocument6 pagesGlycerol MethodsSeptiani RiaNo ratings yet
- Glycerol MethodsDocument6 pagesGlycerol MethodsSeptiani RiaNo ratings yet
- Dec2012 462181Document24 pagesDec2012 462181SyShobanaNo ratings yet
- Value-Added Utilization of Crude Glycerol From BiodieselDocument16 pagesValue-Added Utilization of Crude Glycerol From BiodieselKaweepoj SiwanartnusornNo ratings yet
- Authentication of Questioned Documents Using HPLCDocument19 pagesAuthentication of Questioned Documents Using HPLCab-azzehNo ratings yet
- Back Forward Main Menu TOC Study Guide TOC Textbook Website MHHE WebsiteDocument38 pagesBack Forward Main Menu TOC Study Guide TOC Textbook Website MHHE Websiteauguste noeNo ratings yet
- Common unit cells and crystal structuresDocument99 pagesCommon unit cells and crystal structuresasjfgauojfgfNo ratings yet
- UV-Vis Analysis of Iron in WaterDocument5 pagesUV-Vis Analysis of Iron in WaterCleiton Tavares PessoaNo ratings yet
- Chem T8 HLQDocument27 pagesChem T8 HLQSimar ChadhaNo ratings yet
- Chemistry CH No 10Document5 pagesChemistry CH No 10Syed Salman SaeedNo ratings yet
- ThesisDocument94 pagesThesisSarah LimNo ratings yet
- Caustic Recovery Tests ReportDocument17 pagesCaustic Recovery Tests ReportrashidafmNo ratings yet
- DSP Seminar 2 McCabe-Thiele Method Column FeedDocument4 pagesDSP Seminar 2 McCabe-Thiele Method Column Feedanto3harrish3fdoNo ratings yet
- Distillation: C H E 2 4 6 Separation ProcessDocument41 pagesDistillation: C H E 2 4 6 Separation ProcessnorazifahNo ratings yet
- Testing Water Quality: pH and Dissolved Oxygen LevelsDocument5 pagesTesting Water Quality: pH and Dissolved Oxygen LevelsIris BercesNo ratings yet
- Assay of Antimony Potassium TartrateDocument2 pagesAssay of Antimony Potassium TartrateTricia So100% (3)
- Lecture-Unit 9 Chemical EquilibriumDocument13 pagesLecture-Unit 9 Chemical EquilibriumKemoy FrancisNo ratings yet
- LC-MS-MS Education Series - Quadrupole Theory and UseDocument2 pagesLC-MS-MS Education Series - Quadrupole Theory and UseLuis MartinezNo ratings yet
- Theories of Acids and Bases: Beirut Arab University Faculty of Science Debbieh CampusDocument19 pagesTheories of Acids and Bases: Beirut Arab University Faculty of Science Debbieh CampusMoh AmmNo ratings yet
- High Pressure Boiler Water TreatmentDocument2 pagesHigh Pressure Boiler Water TreatmentTeAnaruNo ratings yet
- Report Data LoggerDocument3 pagesReport Data LoggerIntan NoranizaNo ratings yet
- Genchem 2 W7Document6 pagesGenchem 2 W7Christian PazNo ratings yet
- 1-4 Concentrate On Keeping Up Your StandardsDocument21 pages1-4 Concentrate On Keeping Up Your Standardsmaryamshahzad489No ratings yet
- Chemistry & Chemical Engineering CatalogDocument100 pagesChemistry & Chemical Engineering CatalogLuigi BoeriNo ratings yet
- Thin Layer Chromatography Lab Report Experiment 04Document5 pagesThin Layer Chromatography Lab Report Experiment 04PDPPPMAT0621 Ruhilin Binti NasserNo ratings yet
- 110 Lab 12 TitrationDocument8 pages110 Lab 12 TitrationShasvat JainNo ratings yet
- High Manganese Steel Certified Reference MaterialDocument2 pagesHigh Manganese Steel Certified Reference Materiallehdruk7100No ratings yet
- Fenofibrate EP 11.0Document2 pagesFenofibrate EP 11.0noschNo ratings yet
- 53-CG 244 730Document22 pages53-CG 244 730misulica80No ratings yet
- Heuristic Synthesis and Shortcut Design of Separation Processes Using Residue Curve Maps - A ReviewDocument18 pagesHeuristic Synthesis and Shortcut Design of Separation Processes Using Residue Curve Maps - A ReviewFDNo ratings yet
- Planner Chromatography by Miss IshratDocument13 pagesPlanner Chromatography by Miss IshratTuba AhmedNo ratings yet
- Methods of RRLDocument4 pagesMethods of RRLhelloNo ratings yet
- Laboratory Outline - Exercise 5Document6 pagesLaboratory Outline - Exercise 5Majestic RavenNo ratings yet
- MSC ChemistryDocument54 pagesMSC ChemistryShivam Vinoth100% (1)