Professional Documents
Culture Documents
Protein Sorting and Transport:: The Endoplasmic Reticulum, Golgi Apparatus, and Lysosomes
Uploaded by
Srikant Singh0 ratings0% found this document useful (0 votes)
7 views99 pagesupload file
Original Title
Ch10 Lecture
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentupload file
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views99 pagesProtein Sorting and Transport:: The Endoplasmic Reticulum, Golgi Apparatus, and Lysosomes
Uploaded by
Srikant Singhupload file
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 99
10
Protein Sorting and Transport:
The Endoplasmic Reticulum, Golgi Apparatus,
and Lysosomes
10 Introduction
• In addition to the presence of a nucleus,
eukaryotic cells are distinguished from prokaryotic
cells by the presence of membrane-enclosed
organelles within their cytoplasm.
• Many proteins destined for the endoplasmic
reticulum, the Golgi apparatus, lysosomes, the
plasma membrane, and secretion from the cell
are synthesized on ribosomes that are bound to
the membrane of the endoplasmic reticulum.
10 The Endoplasmic Reticulum
• The endoplasmic reticulum, or ER, is a network of
membrane-enclosed tubules and sacs that
extends from the nuclear membrane throughout
the cytoplasm.
• The rough ER is covered by ribosomes on its
outer surface and is involved in protein
metabolism.
10.1 The endoplasmic reticulum (ER)
10 The Endoplasmic Reticulum
• The transitional ER is involved in protein
processing and is where vesicles exit to the Golgi
apparatus.
• The smooth ER is involved in lipid, rather than
protein, metabolism and is not associated with
ribosomes.
10 The Endoplasmic Reticulum and Protein Secretion
• The endoplasmic reticulum plays a key role in
protein processing and sorting.
• Secretory vesicles carry radiolabeled proteins
from the Golgi apparatus to the cell surface, then
fuse with the plasma membrane to release their
contents outside of the cell.
10.2 The secretory pathway
10 The Endoplasmic Reticulum and Protein Secretion
• The secretory pathway consists of the rough ER
→ Golgi → secretory vesicles → cell exterior.
• The entrance of proteins into the ER represents a
major branch point for the traffic of proteins within
eukaryotic cells.
10.3 Overview of protein sorting
10 Targeting Proteins to the Endoplasmic Reticulum
• Proteins can be translocated into the ER either
during their synthesis on membrane-bound
ribosomes or after their translation has been
completed on free ribosomes in the cytosol.
• A signal sequence targets ribosomes, engaged in
the synthesis of proteins that are destined for
secretion, to the endoplasmic reticulum.
10.4 Isolation of rough ER
10 Targeting Proteins to the Endoplasmic Reticulum
• Microsomes are small vesicles formed from the
endoplasmic reticulum when cells are disrupted.
• The signal for ribosome attachment to the ER
might be an amino acid sequence near the amino
terminus of the growing polypeptide chain.
10.5 Incorporation of secretory proteins into microsomes
10 Targeting Proteins to the Endoplasmic Reticulum
• It has been hypothesized and substantiated that
an amino terminal leader sequence targets the
polypeptide chain to the microsomes and is then
cleaved by a microsomal protease.
• The mechanism by which secretory proteins are
targeted to the ER during their translation consists
of signal sequences that span about 20 amino
acids.
10.6 The signal sequence of growth hormone
10 Targeting Proteins to the Endoplasmic Reticulum
• Signal recognition particles, or SRPs, recognize
and bind to signal sequences as the latter emerge
from the ribosome.
• Small cytoplasmic RNAs, or srpRNAs, are
components of SRP.
10.7 Structure of the SRP
10 Targeting Proteins to the Endoplasmic Reticulum
• SRP receptors are proteins on the membrane of
the endoplasmic reticulum that bind the signal
recognition particle, SRP.
• A translocon is a membrane channel through
which polypeptide chains are transported into the
endoplasmic reticulum.
10.8 Cotranslational targeting of secretory proteins to the ER
10 Targeting Proteins to the Endoplasmic Reticulum
• Yeast and mammalian translocon proteins are
closely related to the plasma membrane proteins
that translocate secreted polypeptides in bacteria.
• Signal peptidase cleaves the signal sequence and
releases it into the lumen of the ER.
10 Targeting Proteins to the Endoplasmic Reticulum
• Many proteins in yeast are targeted to the ER
after their translation is complete rather than
being transferred into the ER during synthesis on
membrane-bound ribosomes.
10.9 Posttranslational translocation of proteins into the ER
10 Insertion of Proteins into the ER Membrane
• Proteins destined for secretion from the cell or
residence within the lumen of the ER, Golgi
apparatus, endosomes, or lysosomes are
translocated across the ER membrane and
released into the lumen of the ER.
• Integral membrane proteins are embedded in the
membrane by hydrophobic sequences that span
the phospholipid bilayer.
10.10 Orientations of membrane proteins
10 Insertion of Proteins into the ER Membrane
• The lumen of the ER is topologically equivalent to
the exterior of the cell, so the domains of plasma
membrane proteins that are exposed on the cell
surface correspond to the regions of polypeptide
chains that are translocated into the ER lumen.
• The most straightforward mode of insertion into
the ER membrane results in the synthesis of
transmembrane proteins oriented with their
carboxy termini exposed to the cytosol.
10.11 Topology of the secretory pathway
10.12 Membrane protein with a cleavable signal sequence and a single stop-transfer sequence
10 Insertion of Proteins into the ER Membrane
• Proteins can also be anchored in the ER
membrane by internal signal sequences that are
not cleaved by signal peptidase.
• Proteins that span the membrane multiple times
are thought to be inserted as a result of an
alternating series of internal signal sequences and
transmembrane stop-transfer sequences.
10.13 Insertion of membrane proteins with internal noncleavable signal sequences (Part 1)
10.13 Insertion of membrane proteins with internal noncleavable signal sequences (Part 2)
10.14 Insertion of a protein that spans the membrane multiple times
10 Insertion of Proteins into the ER Membrane
• Most transmembrane proteins destined for other
compartments in the secretory pathway are
delivered to them in transport vesicles.
10 Protein Folding and Processing in the ER
• For proteins that enter the secretory pathway,
many of these events occur either during
translocation across the ER membrane or within
the ER lumen.
• After proteins are translocated across the ER
membrane as unfolded polypeptide chains, they
fold into their three-dimensional conformations,
assisted by molecular chaperones.
10.15 Protein folding in the ER
10 Protein Folding and Processing in the ER
• Protein disulfide isomerase is located in the ER
lumen and is an enzyme that facilitates disfulfide
bond formation.
• Glycosylphosphatidylinositol, or GPI anchors, are
membrane-anchoring glycolipids that contain
phosphatidylinositol.
10.16 Protein glycosylation in the ER
10.17 Addition of GPI anchors
10 Quality Control in the ER
• Many proteins synthesized in the ER are rapidly
degraded, primarily because they fail to fold
correctly; others reside in the ER for several hours
while they are properly folded.
• The unfolded protein response is a cellular stress
reponse in which an excess of unfolded proteins
in the endoplasmic reticulum leads to general
inhibition of protein synthesis, increased
expression of chaperones, and increased
proteasome activity.
10.18 Glycoprotein folding by calnexin
10.19 Unfolded protein response (Part 1)
10.19 Unfolded protein response (Part 2)
10 The Smooth ER and Lipid Synthesis
• Because they are extremely hydrophobic,
membrane lipids are synthesized in association
with already existing cellular membranes rather
than in the aqueous environment of the cytosol.
• The membranes of eukaryotic cells are composed
of three main types of lipids: phospholipids,
glycolipids, and cholesterol.
10.20 Synthesis of phospholipids
10.20 Synthesis of phospholipids (Part 1)
10.20 Synthesis of phospholipids (Part 2)
10 The Smooth ER and Lipid Synthesis
• The synthesis of phospholipids on the cytosolic
side of the ER membrane allows the hydrophobic
fatty acid chains to remain buried in the
membrane while membrane-bound enzymes
catalyze their reactions with water-soluble
precursors in the cytosol.
• Flippases are membrane proteins that catalyze
the rapid translocation of phospholipids across the
ER membrane resulting in even growth of both
halves of the bilayer.
10.21 Translocation of phospholipids across the ER membrane
10 The Smooth ER and Lipid Synthesis
• In addition to the role in synthesis of the glycerol
phospholipids, the ER also serves as the major
site of synthesis of two other membrane lipids:
cholesterol and ceramide.
• Smooth ER is abundant in cell types that are
particularly active in lipid metabolism.
10.22 Structure of cholesterol and ceramide
10 Export of Proteins and Lipids from the ER
• Both proteins and phospholipids travel along the
secretory pathway in transport vesicles, which
bud from the membrane of one organelle and
then fuse with the membrane of another.
• Most proteins that enter the transitional ER move
through the ER-Golgi intermediate compartment
and on to the Golgi.
10.23 Vesicular transport from the ER to the Golgi
10.24 ER export signals
10 Export of Proteins and Lipids from the ER
• If proteins that function within the ER are allowed
to proceed along the secretory pathway, they will
be lost to the cell.
• KDEL and KKXX signals do not prevent ER
proteins from being packaged into vesicles and
carried to the Golgi; instead they cause these ER
resident proteins to be selectively retrieved from
the ER-Golgi intermediate compartment or the
Golgi complex and returned to the ER via a
recycling pathway.
10.25 Retrieval of resident ER proteins
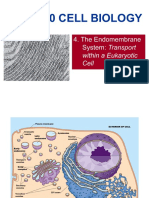
10 The Golgi Apparatus
• The Golgi apparatus, or Golgi complex, functions
as a factory in which proteins received from the
ER are further processed and sorted for transport
to their eventual destinations—endosomes,
lysosomes, the plasma membrane, or secretion
from the cell.
• In plant cells, the Golgi apparatus further serves
as the site at which the complex polysaccharides
of the cell wall are synthesized.
10 Organization of the Golgi
• In most cells, the Golgi is composed of flattened
membrane-enclosed sacs and associated
vesicles.
• The cis Golgi network is the region of the Golgi
apparatus at which proteins enter from the
endoplasmic reticulum.
10.26 Electron micrograph of a Golgi apparatus
10 Organization of the Golgi
• The Golgi stack consists of the compartments of
the Golgi apparatus within which most metabolic
activities take place.
• The trans Golgi network is the Golgi compartment
within which proteins are sorted and packaged to
exit the Golgi apparatus.
• The mechanism by which proteins move through
the Golgi apparatus has still not been established
and is an area of controversy among cell
biologists.
10.27 Regions of the Golgi apparatus
10 Protein Glycosylation within the Golgi
• Protein processing within the Golgi involves the
modification and synthesis of the carbohydrate
portions of glycoproteins.
• N-linked oligosaccharides are processed within
the Golgi apparatus in an ordered sequence of
reactions.
10.28 Processing of N-linked oligosaccharides in the Golgi
10 Protein Glycosylation within the Golgi
• A glycosyltransferase is an enzyme that adds
sugar residues to its substrate.
• A glycosidase is an enzyme that removes sugar
residues from its substrate.
• The processing of the N-linked oligosaccharide of
lysosomal proteins differs from that of secreted
and plasma membrane proteins.
10.29 Targeting of lysosomal proteins by phosphorylation of mannose residues
10 Protein Glycosylation within the Golgi
• Mannose-6-phosphate residues are what is left on
the N-linked oligosaccharide after N-
acetylglucosamine is removed.
• Signal patches are recognition determinants
formed by the three-dimensional folding of a
polypeptide chain.
10 Lipid and Polysaccharide Metabolism in the Golgi
• In addition to its activities in processing and
sorting glycoproteins, the Golgi apparatus
functions in lipid metabolism—in particular, in the
synthesis of glycolipids and sphingomyelin.
• Sphingomyelin is synthesized on the lumenal
surface of the Golgi, but glucose is added to
ceramide on the cytosolic side.
• In plant cells, the Golgi apparatus has the
additional task of serving as the site where
complex polysaccharides of the cell wall are
synthesized.
10.30 Synthesis of sphingomyelin and glycolipids
10 Protein Sorting and Export from the Golgi
Apparatus
• Proteins as well as lipids and polysaccharides are
transported from the Golgi apparatus to their final
destinations through the secretory pathway.
• The constitutive secretory pathway, which
operates in all cells, leads to continual
unregulated protein secretion.
10.31 Transport from the Golgi apparatus
10 Protein Sorting and Export from the Golgi
Apparatus
• The apical domain is the exposed free surface of
a polarized epithelial cell.
• The basolateral domain is the surface region of a
polarized epithelial cell that is in contact with
adjacent cells or the extracellular matrix.
• The apical membrane of intestinal epithelial cells
faces the lumen of the intestine and is specialized
for the efficient absorption of nutrients; the
remainder of the cell is covered by the basolateral
membrane.
10.32 Transport to the plasma membrane of polarized cells
10 Protein Sorting and Export from the Golgi
Apparatus
• A vacuole is a large membrane-enclosed sac in
the cytoplasm of eukaryotic cells.
In
plant cells, vacuoles function to store
nutrients and waste products, to degrade
macromolecules, and to maintain turgor
pressure.
10.33 A plant cell vacuole
10 The Mechanism of Vesicular Transport
• Vesicular transport is a major cellular activity,
responsible for molecular traffic between a variety
of specific membrane-enclosed compartments.
• The selectivity of such transport is key to
maintaining the functional organization of the cell.
10 Experimental Approaches to Understanding
Vesicular Transport
• Progress toward elucidating the molecular
mechanisms of vesicular transport has been
made by several distinct experimental
approaches.
• Biochemical studies of vesicular transport using
reconstituted systems have complemented
genetic studies and have enabled the direct
isolation of transport proteins from mammalian
cells.
• Synaptic vesicles are secretory vesicles that
release neurotransmitters at a synapse.
10 Cargo Selection, Coat Proteins, and Vesicle
Budding
• Most transport vesicles that carry secretory
proteins from the ER to the Golgi and from the
Golgi to other targets are coated with cytosolic
coat proteins and thus are called coated vesicles.
• The formation of coated vesicles is regulated by
small GTP-binding proteins related to Ras and
Ran.
10.34 Formation and fusion of a transport vesicle
10 Cargo Selection, Coat Proteins, and Vesicle
Budding
• COP-coated vesicles are transport vesicles
coated with COP I or COP II.
• COP I and COP II are two proteins other than
clathrin that coat transport vesicles.
• Clathrin is a protein that coats the cytoplasmic
surface of cell membranes and assembles into
basketlike lattices that drive vesicle budding.
10.35 Initiation of a clathrin-coated vesicle by ARF1
10.36 Incorporation of lysosomal proteins into clathrin-coated vesicles
10.36 Incorporation of lysosomal proteins into clathrin-coated vesicles (Part 1)
10.36 Incorporation of lysosomal proteins into clathrin-coated vesicles (Part 2)
10 Vesicle Fusion
• The fusion of a transport vesicle with its target
involves two types of events.
• The SNARE hypothesis states that vesicle fusion
is mediated by pairs of transmembrane proteins,
or SNAREs, on the vesicle and target
membranes.
• The Rab family of small GTP-binding proteins
plays a key role in vesicular transport.
10 Vesicle Fusion
• Individual Rab proteins or combinations of Rab
proteins mark different organelles and transport
vesicles, so their localization on the correct
membrane is key to establishing the specificity of
vesicular transport.
• To initiate transport vesicle fusion, Rab/GTP on
the transport vesicle interacts with effector
proteins and v-SNAREs to assemble a pre-fusion
complex.
10.37 Delivery of Rab to a membrane
10.38 Vesicle fusion
10 Vesicle Fusion
• Membrane fusion is a general process that occurs
whenever a transport vesicle fuses with a target
membrane.
10.39 Exocyst assembly and vesicle targeting
10 Lysosomes
• Lysosomes are membrane-enclosed organelles
that contain an array of enzymes capable of
breaking down all types of biological polymers.
10.40 Electron micrograph of lysosomes and mitochondria in a mammalian cell
10 Lysosomal Acid Hydrolases
• Lysosomal storage diseases are a family of
diseases characterized by the accumulation of
undegraded material in the lysosomes of affected
individuals.
• Most lysosomal enzymes are acid hydrolases,
which are active at the acidic pH that is
maintained within lysosomes but not at the neutral
pH characteristic of the rest of the cytoplasm.
10.41 Organization of the lysosome
10 Endocytosis and Lysosome Formation
• Endocytosis is the uptake of extracellular material
in vesicles formed from the plasma membrane.
• Endosome formation represents an intersection
between the secretory pathway through which
lysosomal proteins are processed, and the
endocytic pathway through which extracellular
molecules are taken up at the cell surface.
• Late endosomes mature into lysosomes as they
acquire a full complement of acid hydrolases,
which digest the molecules originally taken up by
endocytosis.
10.42 Endocytosis and lysosome formation
10.42 Endocytosis and lysosome formation (Part 1)
10.42 Endocytosis and lysosome formation (Part 2)
10 Phagocytosis and Autophagy
• In addition to degrading molecules taken up by
endocytosis, lysosomes digest material derived
from two other routes—phagocytosis and
autophagy.
• In phagocytosis, specialized cells, such as
macrophages, take up and degrade large
particles, including bacteria, cell debris, and aged
cells that need to be eliminated from the body.
• Phagosomes are phagocytic vacuoles that take in
these large particles.
10.43 Lysosomes in phagocytosis and autophagy
10 Phagocytosis and Autophagy
• Phagosomes fuse with lysosomes and become
phagolysosomes.
• The process of autophagy takes place in
lysosomes and is the gradual turnover of the cell’s
own components.
• An autophagosome is a vesicle containing internal
organelles enclosed by fragments of the
endoplasmic reticulum membrane. The
autophagosome fuses with a lysosome and its
contents are digested.
You might also like
- Endomembrane System: The Cell's Internal Transport NetworkDocument26 pagesEndomembrane System: The Cell's Internal Transport NetworkPreeti SainiNo ratings yet
- ER Role in Protein Synthesis and ModificationDocument56 pagesER Role in Protein Synthesis and Modificationkubuldinho88% (8)
- Endomembrane SystemDocument56 pagesEndomembrane SystemSteven Joshua DrizNo ratings yet
- Intracellular TransportDocument66 pagesIntracellular Transportalvitakhoridatul100% (1)
- Without Pics Assignment Protein Sorting and TargetingDocument3 pagesWithout Pics Assignment Protein Sorting and TargetingShaher Bano MirzaNo ratings yet
- Cell Biology-Ch12-Part 2-v2Document30 pagesCell Biology-Ch12-Part 2-v2조형윤No ratings yet
- Endomembrane SystemDocument67 pagesEndomembrane SystemjhanvisNo ratings yet
- Protein SortingDocument13 pagesProtein Sortingdkshukla79100% (4)
- Membrane Biogenesis lms2021Document39 pagesMembrane Biogenesis lms2021Omowunmi EmmanuelNo ratings yet
- Lecture 2: Protein Sorting (Endoplasmic Reticulum) : Dr. Diala Abu-Hsasan School of MedicineDocument33 pagesLecture 2: Protein Sorting (Endoplasmic Reticulum) : Dr. Diala Abu-Hsasan School of MedicineAhmad DamatiNo ratings yet
- Protein Sorting: Dr. Narendhirakannan RT Assistant Professor Department of BiochemistryDocument43 pagesProtein Sorting: Dr. Narendhirakannan RT Assistant Professor Department of Biochemistryمروة صلاح100% (1)
- 19.5 Protein Targeting and SortingDocument9 pages19.5 Protein Targeting and SortingMacy MarianNo ratings yet
- Membranes Part 2 StudentDocument55 pagesMembranes Part 2 StudentBrownyNo ratings yet
- Endoplasmic ReticulumDocument3 pagesEndoplasmic ReticulumHanumat SinghNo ratings yet
- Mechanism of Intracellular Compartments and Protein Sorting2Document6 pagesMechanism of Intracellular Compartments and Protein Sorting2ahmeddiab2022No ratings yet
- Capítulo 13Document61 pagesCapítulo 13AlejandraNo ratings yet
- PL4 Cell CompartmentsDocument11 pagesPL4 Cell CompartmentsJake GopitaNo ratings yet
- References: P. 495-498Document63 pagesReferences: P. 495-498Ciania KimNo ratings yet
- Structure and Functions of Eukaryotic OrganellesDocument44 pagesStructure and Functions of Eukaryotic OrganellesChristineNo ratings yet
- Reticulum Endoplasmicum AnsDocument34 pagesReticulum Endoplasmicum AnsNurul MuthiahNo ratings yet
- Biochemistry & Genetics: II SHS 109: Resource Person: DR Tanveer Akbar Reference TextDocument60 pagesBiochemistry & Genetics: II SHS 109: Resource Person: DR Tanveer Akbar Reference TexttNo ratings yet
- 4, 5 - The Cytoplasm - 2Document58 pages4, 5 - The Cytoplasm - 2Muthana Bani YassenNo ratings yet
- Intracellular Compartment and Protein Sorting RoadmapDocument33 pagesIntracellular Compartment and Protein Sorting RoadmapHaddhi WhibowoNo ratings yet
- 1. Cell 1Document47 pages1. Cell 1JeniNo ratings yet
- Cell HandoutsDocument25 pagesCell Handoutsgameaus00No ratings yet
- Membrane Structure and the Fluid Mosaic ModelDocument7 pagesMembrane Structure and the Fluid Mosaic ModelHyunji KimNo ratings yet
- Fronda - CMB - PPT - Endoplasmic ReticulumDocument45 pagesFronda - CMB - PPT - Endoplasmic ReticulumJericho D. FrondaNo ratings yet
- Smooth and Granular Endoplasmic Reticulum RibosomesDocument26 pagesSmooth and Granular Endoplasmic Reticulum RibosomesArfiNo ratings yet
- Geral Aula #2b - Bioquimica e Biologia CelularDocument39 pagesGeral Aula #2b - Bioquimica e Biologia CelularsusanajpNo ratings yet
- Intra Cellular Vesicular TrafficDocument48 pagesIntra Cellular Vesicular TrafficKanishkaNo ratings yet
- Biology - Smooth & Rough EDocument12 pagesBiology - Smooth & Rough ESandhyaPremNo ratings yet
- Chapter 12Document26 pagesChapter 12Macy MarianNo ratings yet
- Lipids, Membranes and Transport OutlineDocument25 pagesLipids, Membranes and Transport Outlinefalcons22No ratings yet
- Zoology CHDocument6 pagesZoology CHAtika SadafNo ratings yet
- Pale Child with Splenomegaly and Hemolytic AnemiaDocument49 pagesPale Child with Splenomegaly and Hemolytic AnemiaAwais RehmanNo ratings yet
- Eukaryote Translation PDFDocument21 pagesEukaryote Translation PDFChandra Mohan Meena100% (1)
- Intracellular Compartments and Protein SortingDocument55 pagesIntracellular Compartments and Protein SortingTIGER BHAINo ratings yet
- Protein TransportDocument13 pagesProtein TransportvmshanesNo ratings yet
- Membrane Topology, Protein Trafficking and the ERDocument46 pagesMembrane Topology, Protein Trafficking and the ERbrian8576No ratings yet
- Protein Structure, Targeting and SortingDocument28 pagesProtein Structure, Targeting and SortingmskikiNo ratings yet
- Cell Theory and Biochemical Aspects of Cell MembraneDocument39 pagesCell Theory and Biochemical Aspects of Cell MembraneHaroon BadarNo ratings yet
- Basic Cell Structure & FunctionDocument27 pagesBasic Cell Structure & FunctionJawaad AsifNo ratings yet
- Protein TargetingDocument10 pagesProtein TargetingdwigusmalawatiNo ratings yet
- Cell 1Document34 pagesCell 1Majd HusseinNo ratings yet
- Cells Structure & FunctionDocument80 pagesCells Structure & Functionderr barrNo ratings yet
- ER Presentation by Abhay AryaDocument17 pagesER Presentation by Abhay AryaAbhay AryaNo ratings yet
- Er, Structure & Its FunctionsDocument20 pagesEr, Structure & Its FunctionsTalha AfzalNo ratings yet
- Structure of Endoplasmic Reticulum (ER)Document8 pagesStructure of Endoplasmic Reticulum (ER)Ishita KumariNo ratings yet
- Endoplasmic ReticulumDocument30 pagesEndoplasmic ReticulumMuhammad Junaid Iqbal100% (1)
- Biosynthesis, Modification, and Cell Secretion Track: Endoplasmic Reticulum, Ribosome, and Golgi ComplexDocument5 pagesBiosynthesis, Modification, and Cell Secretion Track: Endoplasmic Reticulum, Ribosome, and Golgi ComplexSri HayuniNo ratings yet
- Internal Organization: Eukaryotic Cells Have They Performs Specific Functions For The CellDocument12 pagesInternal Organization: Eukaryotic Cells Have They Performs Specific Functions For The CellHACKER MANNo ratings yet
- BIOL2120 4 Endomembrane SystemDocument47 pagesBIOL2120 4 Endomembrane SystemHui Ka HoNo ratings yet
- L7.8.9.Cell BiologyDocument81 pagesL7.8.9.Cell BiologyGeethanjali SivakumarNo ratings yet
- Lec 2Document12 pagesLec 2HACKER MANNo ratings yet
- Endoplasmic ReticulumDocument12 pagesEndoplasmic ReticulumAzka AltafNo ratings yet
- Cell Biology: Parts, Functions and CharacteristicsDocument25 pagesCell Biology: Parts, Functions and CharacteristicsVjay PayumoNo ratings yet
- Protein Targetting and Trafficking: How Cells Transport Newly Synthesized Proteins to Their Proper LocationsDocument13 pagesProtein Targetting and Trafficking: How Cells Transport Newly Synthesized Proteins to Their Proper LocationssoundaryaNo ratings yet
- Exosomes: A Clinical CompendiumFrom EverandExosomes: A Clinical CompendiumLawrence R. EdelsteinNo ratings yet
- Sinovac JournalDocument10 pagesSinovac JournalGeulissa AddiniNo ratings yet
- Phase 0: Phases of Clinical TrialsDocument2 pagesPhase 0: Phases of Clinical TrialsSrikant SinghNo ratings yet
- Development and Testing of VaccinesDocument4 pagesDevelopment and Testing of VaccinesSrikant SinghNo ratings yet
- TheCOVID 19vaccinedevelopmentlandscapeDocument3 pagesTheCOVID 19vaccinedevelopmentlandscapelisnerisNo ratings yet
- Asimov Quick MathsDocument190 pagesAsimov Quick MathsDani Ibrahim100% (2)
- Netresult Dec14Document12 pagesNetresult Dec14paramjeet99No ratings yet
- G1-G12 Are Plasmid Samples, M Is Lamda Dna Digested With Hindiii and EcoriDocument1 pageG1-G12 Are Plasmid Samples, M Is Lamda Dna Digested With Hindiii and EcoriSrikant SinghNo ratings yet
- G1-G12 Are Plasmid Samples, M Is Lamda Dna Digested With Hindiii and EcoriDocument1 pageG1-G12 Are Plasmid Samples, M Is Lamda Dna Digested With Hindiii and EcoriSrikant SinghNo ratings yet
- Cell Parts Practice QuizDocument3 pagesCell Parts Practice QuizRodel AzaresNo ratings yet
- Exploring Creation With Biology Schedule For 2013-2014Document17 pagesExploring Creation With Biology Schedule For 2013-2014karen100% (2)
- Chapter 3 - Cells & TissuesDocument197 pagesChapter 3 - Cells & TissuesSean Vladimir SorianoNo ratings yet
- Cell City Introduction!: Biology AnalogiesDocument5 pagesCell City Introduction!: Biology AnalogiesNei chimNo ratings yet
- Pharmacy Faculty Midterm Exam Human Physiology 1Document1 pagePharmacy Faculty Midterm Exam Human Physiology 1starvationNo ratings yet
- Crop Sci Notes 2018 Gwanzura - 063350Document274 pagesCrop Sci Notes 2018 Gwanzura - 063350Evelyn Kanengoni100% (2)
- Organelle Biogenesis ILOs and ReferencesDocument3 pagesOrganelle Biogenesis ILOs and ReferencesDarren MohNo ratings yet
- 9 Science Ncert Ch5Document6 pages9 Science Ncert Ch5harish sharmaNo ratings yet
- The Generalized Animal CellDocument35 pagesThe Generalized Animal CellMelina MarinNo ratings yet
- School Cell Analogy PpointDocument14 pagesSchool Cell Analogy Ppointapi-265180883No ratings yet
- October 2016 (IAL) QP - Unit 2 Edexcel BiologyDocument24 pagesOctober 2016 (IAL) QP - Unit 2 Edexcel BiologyEricka AlvarezNo ratings yet
- Seba BiologyDocument114 pagesSeba BiologymwansaNo ratings yet
- Bioloy Notes From NCERT (Mahendra Coaching NotesDocument385 pagesBioloy Notes From NCERT (Mahendra Coaching NotesBabu VermaNo ratings yet
- Guyton Hall Medical Physiology 13th Test BankDocument5 pagesGuyton Hall Medical Physiology 13th Test BankOsman Nazir33% (3)
- Cell Structure SEDocument7 pagesCell Structure SENithya Majeti100% (1)
- Cell Analogy ScriptDocument3 pagesCell Analogy ScriptCloudetteMendozaNo ratings yet
- Cells: Molecules and Mechanisms (Official)Document283 pagesCells: Molecules and Mechanisms (Official)Axolotl Academic Publishing CoNo ratings yet
- Biochemistry Lec - Prelim TransesDocument20 pagesBiochemistry Lec - Prelim TransesLOUISSE ANNE MONIQUE L. CAYLONo ratings yet
- Keystone Biology ReviewDocument53 pagesKeystone Biology ReviewTalia GelmanNo ratings yet
- The Cellular Level of Organization - AnaphyDocument12 pagesThe Cellular Level of Organization - AnaphyJean Rose SalahayNo ratings yet
- Magic School Bus EssayDocument3 pagesMagic School Bus Essayapi-311220565No ratings yet
- 7.1-7.2 Review KeyDocument9 pages7.1-7.2 Review KeyirwindeepsinghNo ratings yet
- Biology Unitwise MCQ - S (MCAT)Document41 pagesBiology Unitwise MCQ - S (MCAT)M Noaman Akbar100% (1)
- Mcqs in Physiology: Collected By: Professor Bassam Talib Al-Gailani (M.B.CH.B., PHD)Document417 pagesMcqs in Physiology: Collected By: Professor Bassam Talib Al-Gailani (M.B.CH.B., PHD)khywh2qnsrNo ratings yet
- ACTIVITY BIOLOGY Cells Exploration Activities PDFDocument16 pagesACTIVITY BIOLOGY Cells Exploration Activities PDFJoy Fernandez0% (1)
- 2016 Practice Qs For BMS1021 Wk1-6 PDFDocument29 pages2016 Practice Qs For BMS1021 Wk1-6 PDFaskldhfdasjkNo ratings yet
- NCMC103 Prelims ReviewerDocument13 pagesNCMC103 Prelims ReviewerVeronica ShaneNo ratings yet
- ADM MELC2 Cell Structure Quarter1STEM BIO11Document28 pagesADM MELC2 Cell Structure Quarter1STEM BIO11c- msNo ratings yet
- Chapter 01Document40 pagesChapter 01javed iqbal KhanNo ratings yet
- Cell Structure QuestionsDocument16 pagesCell Structure QuestionsDila OzdolNo ratings yet