Professional Documents
Culture Documents
Matter Study Guide For Turboquest
Uploaded by
api-267947714Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Matter Study Guide For Turboquest
Uploaded by
api-267947714Copyright:
Available Formats
Matter Study Guide
Background on Matter:
Atoms- Building Blocks, the smallest
unit of an element
Molecules- a group of two or more
atoms that are joined chemically
What Is Matter:
Matter is anything that has mass and
takes up space
Matter is everything around you
Molecules and atoms are composed of
matter
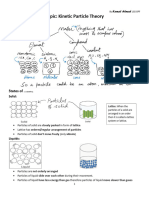
States of Matter:
Solids, liquids, and gases
Also known as phases
Solids- hard and brittle, compact
molecule, vibrate against each other
Liquids- fluidy, fill containers,
Gases- molecules are far apart
Physical Change of Matter:
When molecules move from one phase
to another they are still the same
substance.
Things only move from one phase to
another by physical means. If energy
is added (like increasing the
temperature) or if energy is taken
away (like freezing something), you
have created a physical change.
CHEMISTRY TERM PHASE CHANGE
Fusion/Melting
Freezing
Vaporization/Boiling
Condensation
Sublimation
Deposition
Solid to Liquid
Liquid to Solid
Liquid to Gas
Gas to Liquid
Solid to Gas
Gas to Solid
Fun Fact:
Plasma (a state of matter) was a new
idea when it was identified by William
Crookes in 1879.
You might also like
- The Phases of Matter - Chemistry Book Grade 1 | Children's Chemistry BooksFrom EverandThe Phases of Matter - Chemistry Book Grade 1 | Children's Chemistry BooksNo ratings yet
- Matter Solid Liquid Gas: Page - 1Document5 pagesMatter Solid Liquid Gas: Page - 1Anonymous l309xEA6No ratings yet
- Matter: Definition & The Five States of Matter: AtomsDocument10 pagesMatter: Definition & The Five States of Matter: AtomsJeonila Margarette RapadaNo ratings yet
- Brown Aesthetic Group Project Presentation 20240207 082108 0000Document30 pagesBrown Aesthetic Group Project Presentation 20240207 082108 0000jillianaynemanaNo ratings yet
- Matter, Properties, & Phases: by Emmanuel DikolelayDocument33 pagesMatter, Properties, & Phases: by Emmanuel DikolelayMaPhi ZaBeNo ratings yet
- Notes Kinetic Molecular TheoryDocument4 pagesNotes Kinetic Molecular TheoryGino Carlos MiguelNo ratings yet
- CHEMISTRY SPM FORM 4 Short Notes Chapter 2 THE STRUCTURE OF THE ATOMDocument11 pagesCHEMISTRY SPM FORM 4 Short Notes Chapter 2 THE STRUCTURE OF THE ATOMJay Bee83% (29)
- The Nature of MatterDocument26 pagesThe Nature of MatterEsma KurtanovićNo ratings yet
- Theme: Matter Around Us Learning Area: 2. The Structure of The AtomDocument11 pagesTheme: Matter Around Us Learning Area: 2. The Structure of The AtomSemoi Mathew MatonNo ratings yet
- Heat and Matter NotesDocument8 pagesHeat and Matter NotesHwang ElyseNo ratings yet
- Matter IntroductionDocument33 pagesMatter Introductionapi-313400286No ratings yet
- Physical States of MatterDocument33 pagesPhysical States of MatterAminat OmarNo ratings yet
- JYOTI Chemistry IGCSE Revision NotesDocument16 pagesJYOTI Chemistry IGCSE Revision NotesJyoti MeenaNo ratings yet
- Materi Dan Energi PanasDocument11 pagesMateri Dan Energi PanasShintia Karin DiniartiNo ratings yet
- Module 1 - Nature of MatterDocument11 pagesModule 1 - Nature of MatterButter VittoriNo ratings yet
- Physical Science Overview-1Document5 pagesPhysical Science Overview-1api-315431582No ratings yet
- Matter Separation of MixturesDocument10 pagesMatter Separation of Mixtureslura castroNo ratings yet
- 9th Matter in Our Sorounding Notes 2Document4 pages9th Matter in Our Sorounding Notes 2Santosh RaiNo ratings yet
- Stem Lesson 1 Gen ChemDocument23 pagesStem Lesson 1 Gen ChemYvonne CuevasNo ratings yet
- The Particulate Nature of Matter 2Document32 pagesThe Particulate Nature of Matter 2Pritam CainNo ratings yet
- MCQDocument25 pagesMCQSampat AgnihotriNo ratings yet
- Chapter 6 Solid Liquid and GasesDocument36 pagesChapter 6 Solid Liquid and Gasestashnee pillayNo ratings yet
- Grade 7 Science Chapter 5 NotesDocument7 pagesGrade 7 Science Chapter 5 NotesTajiriMollelNo ratings yet
- Solid, Liquid and GasDocument2 pagesSolid, Liquid and GasJana AldourNo ratings yet
- Sublimation Deposition Condensation VaporisationDocument2 pagesSublimation Deposition Condensation Vaporisationjoenediath9345No ratings yet
- How Do Particles Behave in The Four States of Matter?Document34 pagesHow Do Particles Behave in The Four States of Matter?Abdul RahmanNo ratings yet
- Matter Definition & The Five States of MatterDocument2 pagesMatter Definition & The Five States of MatterAceeNo ratings yet
- Science NotesDocument2 pagesScience Notespenakent12No ratings yet
- Children Encyclopedia Chemistry: The World of KnowledgeFrom EverandChildren Encyclopedia Chemistry: The World of KnowledgeRating: 5 out of 5 stars5/5 (3)
- G7 Matter & Its StatesDocument44 pagesG7 Matter & Its Statesgabrielle.nathan.naomiNo ratings yet
- Solids, Liquids and GasesDocument9 pagesSolids, Liquids and GasesBassel ZeitouniNo ratings yet
- Basic Concepts of ChemistryDocument92 pagesBasic Concepts of ChemistryNehaNo ratings yet
- Science ReviewerDocument5 pagesScience ReviewerMitchell CatulongNo ratings yet
- States of MatterDocument4 pagesStates of MatterBertonNo ratings yet
- Chemisty Igcse Updated Till SyllabusDocument97 pagesChemisty Igcse Updated Till Syllabusapi-181176018No ratings yet
- Phase Changes Lesson 2Document18 pagesPhase Changes Lesson 2nadamohey99No ratings yet
- What Is MatterDocument9 pagesWhat Is MatterAdimahNo ratings yet
- Physical ChangesDocument1 pagePhysical ChangesTheresa Piamonte DagohoyNo ratings yet
- States of MatterDocument5 pagesStates of MattertasneemNo ratings yet
- Reviwer 3RD QuarterDocument19 pagesReviwer 3RD QuartermoraledazoesophiaNo ratings yet
- Matter Separation Tech Notes Myp4Document21 pagesMatter Separation Tech Notes Myp4anitNo ratings yet
- Y9 CH 1 & CH 2 NotesDocument10 pagesY9 CH 1 & CH 2 NotesTeck TieNo ratings yet
- Changes of StatesDocument39 pagesChanges of StatesSalemah MeshalNo ratings yet
- Hi GwynDocument3 pagesHi GwynUtopia CeballoNo ratings yet
- Gen ChemmotDocument4 pagesGen Chemmotgrishamcacal19No ratings yet
- Chapter11 1212 Liquids SolidsDocument90 pagesChapter11 1212 Liquids SolidsVirgilio AbellanaNo ratings yet
- CHEM Exam KeiDocument5 pagesCHEM Exam KeiMaynard NoNo ratings yet
- 5 CH 2 Sec 2 Changes in State and Energy - UploadDocument74 pages5 CH 2 Sec 2 Changes in State and Energy - Uploadapi-270861823No ratings yet
- 02-08-21 G8 (A) The Particle Nature of MatterDocument4 pages02-08-21 G8 (A) The Particle Nature of MatterJose JeramieNo ratings yet
- Quimica Universidad PRINCIPIANTES TraducidaDocument5 pagesQuimica Universidad PRINCIPIANTES TraducidaAUSTRANo ratings yet
- Kinetic Particle theory-OL-NotesDocument6 pagesKinetic Particle theory-OL-Notesshlaibat13No ratings yet
- Competency 9Document23 pagesCompetency 9Charis RebanalNo ratings yet
- States of MatterDocument6 pagesStates of MatterHadeel IbrahimNo ratings yet
- Reviewer For Envi Sci PrelimsDocument8 pagesReviewer For Envi Sci PrelimsGlenmor QuilalaNo ratings yet
- S2 A Chemistry New Class JuneDocument25 pagesS2 A Chemistry New Class Junethiri hsuNo ratings yet
- Unit 5 States of MatterDocument3 pagesUnit 5 States of MatterWezu heavenNo ratings yet
- Basic ChemistryDocument4 pagesBasic ChemistryEmNo ratings yet