Professional Documents
Culture Documents
More Exp
Uploaded by
Agastya SesariandaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
More Exp
Uploaded by
Agastya SesariandaCopyright:
Available Formats
Diffusion with Heterogeneous Chemical Reaction
2A A2 (instantaneously on the catalytic surface)
Gases A & A2
Gas A
Edge of hyp.gas film Z=0 A A2 Z= Catalytic surface
c A N Ax N Ay N Az + + + t x y z
N Az =0 z
= RA
N Az = cD AB
x A + x A ( N Az + N Bz ) z
B = A2
N Az = N A2
1 2
N Az =
cDAA2 x A 1 1 x A z 2
For constant c DAA2
dx A d 1 =0 dz 1 1 x A dz 2
Integration: 2 ln(1 BC 1 : at z = 0 BC 2 : at z =
1 2
x A ) = C1 z + C 2
xA = xA0 xA = 0
(1
1 2
x A ) = (1 x A0 )
1 2
1 ( z / )
N Az =
2cDAA2
1 ln 1 1 x 2 A0
For Diffusion with Slow Heterogeneous Reaction: At the catalytic surface:
N Az = k1 c A = ck1 x A
therefore
(z / )
BC 2: at z=
xA =
N Az ck1
2 (1 1 xA ) = 1 1 N Az 2 ck1
(1 1 xA0 )1 ( z / ) 2
N Az = 2cD AA2
At steady state, N Az cons tan t :
Unsteady:
1 1 ( N Az / ck1 2 ln 1 1 x 2 A0
c A N Ax N Ay N Az + + + y z t x
c x A N Az + =0 t z
= RA
Diffusion with Slow Homogeneous Reaction A+B AB
Gas A
c A N Ax N Ay N Az + + + t x y z
= RA
Z=0 Liquid B Z=L
N Az + k2cA = 0 z
If A and AB are present in small concentrations:
N Az = cD AB
x A + x A ( N Az + N Bz ) z
thus
N Az = DAB
c A z
DAB
d 2c A + k2c A = 0 z 2
BC 1 : BC 2 :
at z = 0 at z = L
cA = cA0 NAz = 0 or dcA/ dz = 0
Gas Absorption with Chemical Reaction in an Agitated Tank HW1: Derive Eq. 17.4 12 Diffusion and Chemical Reaction Inside A Porous Catalyst: HW2: Derive Eq. 17.6-3 fromTable 18.2 - I
You might also like
- CME 301 - Mass Transfer Lecture 2 - 2 2b. Differential Equations For Steady-State Molecular DiffusionDocument18 pagesCME 301 - Mass Transfer Lecture 2 - 2 2b. Differential Equations For Steady-State Molecular DiffusionNajmul Puda PappadamNo ratings yet
- Mass transfer concentration profilesDocument2 pagesMass transfer concentration profilesDelyana RatnasariNo ratings yet
- Thermo HidroDocument11 pagesThermo HidroMuhammad ShohibiNo ratings yet
- P3 Slide 18-25Document4 pagesP3 Slide 18-25Muhammad Taufiq Ikram 1807124818No ratings yet
- Solved Problems On Mass Transfer PDFDocument12 pagesSolved Problems On Mass Transfer PDFProtim DasNo ratings yet
- Introduction to Mass Transfer ConceptsDocument40 pagesIntroduction to Mass Transfer Conceptsnurul syamimieNo ratings yet
- 常用公式及物理常數Document2 pages常用公式及物理常數colinNo ratings yet
- Desarrollo de Un Determinante Por Menores ComplementariosDocument25 pagesDesarrollo de Un Determinante Por Menores ComplementariosNelly CondoriNo ratings yet
- Trigonometric Identities: Formula Sheet Ee2Fh3Document4 pagesTrigonometric Identities: Formula Sheet Ee2Fh3takazogluNo ratings yet
- Chapter 7b, TorsionDocument14 pagesChapter 7b, TorsionSam N SamNo ratings yet
- Chapter 12Document12 pagesChapter 12StefanPerendijaNo ratings yet
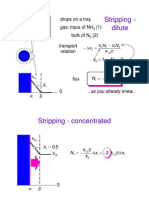
- Stripping - Dilute: Drops On A Tray Gas: Trace of NH (1) Bulk of N (2) Transport RelationDocument9 pagesStripping - Dilute: Drops On A Tray Gas: Trace of NH (1) Bulk of N (2) Transport RelationcymyNo ratings yet
- Lecture3-1 Nuclear MassesDocument24 pagesLecture3-1 Nuclear MassesKaisen JutsuNo ratings yet
- Formula Sheet CME 342Document4 pagesFormula Sheet CME 342Ahmad ElhajNo ratings yet
- Heat and Mass TransferDocument89 pagesHeat and Mass TransferStevo Gadafi BorojevićNo ratings yet
- Molecular Dynamics Simulations of Fluid Flow Boundary ConditionsDocument22 pagesMolecular Dynamics Simulations of Fluid Flow Boundary ConditionsArushi JainNo ratings yet
- CN2125 Heat and Mass Transfer: Dept of Chemical and Biomolecular EngineeringDocument90 pagesCN2125 Heat and Mass Transfer: Dept of Chemical and Biomolecular EngineeringarfpowerNo ratings yet
- Complex Analysis 1988Document26 pagesComplex Analysis 1988Suresh KannanNo ratings yet
- Electromagnetic Waves & Antennas Solutions - 2008Document137 pagesElectromagnetic Waves & Antennas Solutions - 2008DM250% (2)
- Matrices and their inversesDocument5 pagesMatrices and their inversesRam kumarNo ratings yet
- MASS TRANSFER OPERATIONSDocument10 pagesMASS TRANSFER OPERATIONSAnonymous JDXbBDBNo ratings yet
- Correct Série 2Document11 pagesCorrect Série 2khaled sioudNo ratings yet
- Nuclear binding energy and mass calculationsDocument24 pagesNuclear binding energy and mass calculationsKeneth Rey SenyahanNo ratings yet
- Exam 3 SolDocument4 pagesExam 3 SolThierry RodriguesNo ratings yet
- UNIFAC Model for Hexano-Hexylamine MixtureDocument5 pagesUNIFAC Model for Hexano-Hexylamine MixtureJose Rodrigo Quisbert PlataNo ratings yet
- Complex Analysis 1984Document20 pagesComplex Analysis 1984Suresh KannanNo ratings yet
- Math Stats Booklet 1Document20 pagesMath Stats Booklet 1Koh Boon HaoNo ratings yet
- CME 301 - Mass Transfer Differential MT Equations For Unsteady-State Molecular Diffusion (USS-MD)Document24 pagesCME 301 - Mass Transfer Differential MT Equations For Unsteady-State Molecular Diffusion (USS-MD)Najmul Puda PappadamNo ratings yet
- Electrochemistry Assignment: Oxidation-Reduction Reactions and Galvanic CellsDocument5 pagesElectrochemistry Assignment: Oxidation-Reduction Reactions and Galvanic CellsKester Yuree L. GimongalaNo ratings yet
- Linear-Strength Vortex MethodDocument12 pagesLinear-Strength Vortex MethodAshlin AugustyNo ratings yet
- Solving Linear Systems Using MatricesDocument9 pagesSolving Linear Systems Using MatricesGeraldine TimpocNo ratings yet
- Determinants: Intext Exercise: 1Document30 pagesDeterminants: Intext Exercise: 1Remaining TechNo ratings yet
- Determinants ExplainedDocument38 pagesDeterminants ExplainedAvishkar JaiswalNo ratings yet
- PCHM Final Equation SheetDocument3 pagesPCHM Final Equation SheetDanson FanNo ratings yet
- Tutorial 2 - SolutionsDocument29 pagesTutorial 2 - SolutionsVeda Vyas PotnuruNo ratings yet
- Homework Vector SolnDocument7 pagesHomework Vector SolnReemALMousawiNo ratings yet
- Tugas PP Lanjut Shinta Leonita 0906635772Document5 pagesTugas PP Lanjut Shinta Leonita 0906635772HarryNo ratings yet
- FORMULASDocument11 pagesFORMULASXyrus Van Bernard LeomoNo ratings yet
- Sol07 10 PDFDocument4 pagesSol07 10 PDFSidharth VashishtNo ratings yet
- Resolución 1.Document1 pageResolución 1.Elvįş VíąNo ratings yet
- First Order Partial Differential EquationsDocument2 pagesFirst Order Partial Differential EquationsSwapnil JadhavNo ratings yet
- Press 12Document11 pagesPress 12kyamaniderickNo ratings yet
- Diseño Viga LDocument16 pagesDiseño Viga LSergio SotoNo ratings yet
- List MF9: MATHEMATICS (8709, 9709) Higher Mathematics (8719) STATISTICS (0390)Document8 pagesList MF9: MATHEMATICS (8709, 9709) Higher Mathematics (8719) STATISTICS (0390)Muhammad FarhanNo ratings yet
- Fomulario Y Tablas: Istribuciones de Robabilidad Istribuci ONDocument3 pagesFomulario Y Tablas: Istribuciones de Robabilidad Istribuci ONSebastian SandovalNo ratings yet
- 17 Mass Transport - Heterogenous Reaction - WK 12Document25 pages17 Mass Transport - Heterogenous Reaction - WK 12Meesaa KbaiiNo ratings yet
- Summaryof Trigonometric F NDocument3 pagesSummaryof Trigonometric F NAbd El Rahman OmarNo ratings yet
- Complex Variables and Partial Differential Equations SolvedDocument2 pagesComplex Variables and Partial Differential Equations SolvedabcNo ratings yet
- F C D I: Ormulario de Álculo Iferencial E NtegralDocument4 pagesF C D I: Ormulario de Álculo Iferencial E NtegralYaneth hiromishaNo ratings yet
- Matrix Multilplication: Strassen's AlgorithmDocument4 pagesMatrix Multilplication: Strassen's Algorithmstudyonline0212No ratings yet
- Formula Sheet - New Zealand Level 3 Calculus (2009)Document4 pagesFormula Sheet - New Zealand Level 3 Calculus (2009)naedkcinNo ratings yet
- Precal SLM Q2W7-8Document9 pagesPrecal SLM Q2W7-8Anjanette RiparipNo ratings yet
- Equations - Midterm 2Document1 pageEquations - Midterm 2Shawn WongNo ratings yet
- Linear Algebra Determinant2Document34 pagesLinear Algebra Determinant2no nameNo ratings yet
- ME2134 PPT Part 2A PDFDocument14 pagesME2134 PPT Part 2A PDFWai-Yen ChanNo ratings yet
- Formula SheetsDocument51 pagesFormula SheetsHaidee100% (1)
- Instructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYFrom EverandInstructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYNo ratings yet
- Answers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesFrom EverandAnswers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesRating: 1.5 out of 5 stars1.5/5 (2)
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesFrom EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesNo ratings yet
- Vogel's Qualitative Inorganic Analysis PDFDocument356 pagesVogel's Qualitative Inorganic Analysis PDFALCON56YUI88% (24)
- LNF Q200 Brochure 61710Document2 pagesLNF Q200 Brochure 61710Agastya SesariandaNo ratings yet
- LNF Q200 Brochure 61710Document2 pagesLNF Q200 Brochure 61710Agastya SesariandaNo ratings yet
- TrsPh1 2011Document4 pagesTrsPh1 2011Agastya SesariandaNo ratings yet