Professional Documents
Culture Documents
Lecture 5: Alcohols: Rules For Naming Alcohols
Uploaded by
John0 ratings0% found this document useful (0 votes)
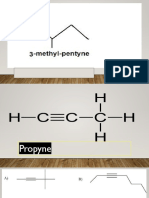
6 views2 pagesAlcohols contain the hydroxyl (-OH) functional group. To name alcohols, identify the longest carbon chain with the -OH group and give it the lowest possible number, then remove the final 'e' and add '-ol'. If there is more than one -OH group, add prefixes like 'di', 'tri' before '-ol'. There are three types of alcohols: primary with one carbon attached to the -OH carbon, secondary with two carbons attached, and tertiary with three carbons attached. Aldehydes contain a carbonyl (-C=O) group that must be at the end of the chain, while ketones also have a carbonyl group but it can
Original Description:
alcohols
Original Title
Lecture 5 Alcohols
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentAlcohols contain the hydroxyl (-OH) functional group. To name alcohols, identify the longest carbon chain with the -OH group and give it the lowest possible number, then remove the final 'e' and add '-ol'. If there is more than one -OH group, add prefixes like 'di', 'tri' before '-ol'. There are three types of alcohols: primary with one carbon attached to the -OH carbon, secondary with two carbons attached, and tertiary with three carbons attached. Aldehydes contain a carbonyl (-C=O) group that must be at the end of the chain, while ketones also have a carbonyl group but it can
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesLecture 5: Alcohols: Rules For Naming Alcohols
Uploaded by
JohnAlcohols contain the hydroxyl (-OH) functional group. To name alcohols, identify the longest carbon chain with the -OH group and give it the lowest possible number, then remove the final 'e' and add '-ol'. If there is more than one -OH group, add prefixes like 'di', 'tri' before '-ol'. There are three types of alcohols: primary with one carbon attached to the -OH carbon, secondary with two carbons attached, and tertiary with three carbons attached. Aldehydes contain a carbonyl (-C=O) group that must be at the end of the chain, while ketones also have a carbonyl group but it can
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Lecture 5: Alcohols
Wednesday, February 17, 2016 1:22 PM
Alcohols are molecules containing the hydroxyl functional group (-OH) .
Rules for naming alcohols
Find the longest chain
containing the OH group. Place
the OH on the lowest possible
number for the chain. Remove
the final e and replace it with -
ol. If there is more than 1 OH
then use di,tri etc before the
ol.
These are some example of alcohols.
Primary alcohol: This is an alcohol in which there is one carbon attached to the carbon with the
OH group e.g propanol.
Secondary alcohol: Where there are two carbons attached to the carbon with the OH group e.g
propan-2-ol.
Organic Chem 1 Page 1
Tertriary alcohol: Where there are 3 carbons attached to the carbon with the OH group e.g 2-
methyl propan-2-ol.
Aldehydes: Are organic compounds which incorporate a carbonyl functional
group ,H- C=O. This functional group must always be at the end of the chain. -al
Ketones: Also consists of the functional group C=O however this can be
located anywhere in the chain. The first 2 ketones are propanone and
butanone.
Organic Chem 1 Page 2
You might also like
- Alcohols, Phenols and EthersDocument28 pagesAlcohols, Phenols and EthersDnyanesh Shinde100% (1)
- The Periodic Table of Elements - Halogens, Noble Gases and Lanthanides and Actinides | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Halogens, Noble Gases and Lanthanides and Actinides | Children's Chemistry BookNo ratings yet
- Alcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"Document33 pagesAlcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"AmanNo ratings yet
- Nomenclature AllDocument83 pagesNomenclature AllLabib HasnainNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Alcohols and Alkyl HalidesDocument13 pagesAlcohols and Alkyl HalidesIRISH REEM LINAOTANo ratings yet
- CBSE Class 12 Alcohol Phenol and Ether Study NotesDocument378 pagesCBSE Class 12 Alcohol Phenol and Ether Study NotesV T PRIYANKANo ratings yet
- 2021 WTS 12 Organic ChemistryDocument56 pages2021 WTS 12 Organic ChemistryGladwell PhetlaNo ratings yet
- Recent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumFrom EverandRecent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumW. D. OllisNo ratings yet
- Test 3 Retrosynthesis and Synthetic Design Practice-AnswersDocument3 pagesTest 3 Retrosynthesis and Synthetic Design Practice-AnswersHelmi Fauzi100% (1)
- Alcohols Esther ThiolsDocument3 pagesAlcohols Esther ThiolsAeva CamachoNo ratings yet
- Chem1-Lec11-Other Organic CompoundsDocument60 pagesChem1-Lec11-Other Organic CompoundsSkud GuillermoNo ratings yet
- 7.1 Alcohols Phenols NomenclatureDocument9 pages7.1 Alcohols Phenols NomenclatureVergil HashimotoNo ratings yet
- Organic Compounds and NomenclatureDocument22 pagesOrganic Compounds and NomenclatureNunaNo ratings yet
- Chem-Report-OlDocument18 pagesChem-Report-OlThean SivaprahasamNo ratings yet
- Hydrocarbonsderivates ObidosDocument12 pagesHydrocarbonsderivates ObidosMa Belle Jasmine DelfinNo ratings yet
- 1.4 Alcohols, Ethers, ThiolsDocument17 pages1.4 Alcohols, Ethers, ThiolsMia PereiraNo ratings yet
- Sanjay Gupta: AlcoholsDocument22 pagesSanjay Gupta: AlcoholsSanjay GuptaNo ratings yet
- Alcohol ChemistryDocument73 pagesAlcohol ChemistryBapu Thorat0% (1)
- Alcohols 2Document4 pagesAlcohols 2Abdullah SalmainNo ratings yet
- Nomenclature - Carbon BondingDocument28 pagesNomenclature - Carbon BondingRobertMurray100% (1)
- FN 4Document4 pagesFN 4idon'tgiveachogiwaNo ratings yet
- Alcohols PresentationDocument9 pagesAlcohols PresentationWanineoNo ratings yet
- 10.2 Alcohols Think IBDocument13 pages10.2 Alcohols Think IBNatalie YoungNo ratings yet
- Oxygen Containing CompoundsDocument15 pagesOxygen Containing Compoundsguia macatangayNo ratings yet
- Topic 5 - AlcoholsDocument7 pagesTopic 5 - AlcoholsRichard WalkerNo ratings yet
- MCAT Organic ChemistryDocument4 pagesMCAT Organic ChemistrySheriffCaitlynNo ratings yet
- Alcohol and HalidesDocument27 pagesAlcohol and HalidesJohn DuranoNo ratings yet
- Chem NotesDocument2 pagesChem Notesayshasarip13No ratings yet
- Chm102 Lec - FinalsDocument21 pagesChm102 Lec - FinalsChurva EklavuNo ratings yet
- Alkohol Dan Eter: Ikatan Tunggal Karbon Ke OksigenDocument34 pagesAlkohol Dan Eter: Ikatan Tunggal Karbon Ke OksigenCoco LemonNo ratings yet
- Alkohol Dan Eter: Ikatan Tunggal Karbon Ke OksigenDocument34 pagesAlkohol Dan Eter: Ikatan Tunggal Karbon Ke OksigenAdamas CarlosNo ratings yet
- 1289 - Alcohol, Phenol EtherDocument37 pages1289 - Alcohol, Phenol EtherKhan DildarNo ratings yet
- Alchohols, Ethers, PhenolsDocument45 pagesAlchohols, Ethers, PhenolsMohammed JunaidNo ratings yet
- AlcoholsDocument22 pagesAlcoholsSai Sasivardhan GampaNo ratings yet
- Experiment 65 Notes: Abbreviations UsedDocument4 pagesExperiment 65 Notes: Abbreviations UsedDIey ChokiEyNo ratings yet
- SCL1 CHMDocument6 pagesSCL1 CHMnini myungNo ratings yet
- Organic Acids Without A Carboxylic Acid Functional GroupDocument6 pagesOrganic Acids Without A Carboxylic Acid Functional GroupkiriokaNo ratings yet
- FCH30806 - Phenolics - Lecture 2 - 2024Document47 pagesFCH30806 - Phenolics - Lecture 2 - 2024matthewlowton2255No ratings yet
- OrganicChemistry NoteDocument16 pagesOrganicChemistry NotetacocatNo ratings yet
- UNIT 2 Organic, Energetics, Kinetics and Equilibrium Part 1Document7 pagesUNIT 2 Organic, Energetics, Kinetics and Equilibrium Part 1Rameez Mazhar SiddiqiNo ratings yet
- Organic 2,3Document22 pagesOrganic 2,3rachit agarwalNo ratings yet
- Compounds Containing OxygenDocument3 pagesCompounds Containing OxygenLouisse Francheska MercadoNo ratings yet
- 11-ORGANIC-CHEMISTRY ImpDocument5 pages11-ORGANIC-CHEMISTRY ImpSandeep PlaysNo ratings yet
- Alcohols Phenols and Ether by AarkumarDocument0 pagesAlcohols Phenols and Ether by AarkumarNikhil Surya MukhiNo ratings yet
- Topic 10 Organic ChemistryDocument74 pagesTopic 10 Organic Chemistryapi-546066323No ratings yet
- Alcohols - Nomenclature and ClassificationDocument10 pagesAlcohols - Nomenclature and ClassificationDionisio BrinosaNo ratings yet
- Different Functional Groups and Their Uses in Organic Compounds 2Document25 pagesDifferent Functional Groups and Their Uses in Organic Compounds 2Belaro JennyNo ratings yet
- 102 CH 15A - Aldehyde and Ketone Structures and NamesDocument16 pages102 CH 15A - Aldehyde and Ketone Structures and NamesGracie OlsonNo ratings yet
- Functional Groups Name, Structure, ReactionDocument48 pagesFunctional Groups Name, Structure, ReactionmichaelmarshallNo ratings yet
- IntnomencarbonylsDocument4 pagesIntnomencarbonylsMiguel Andrew MoralesNo ratings yet
- Organic Chemistry NoteDocument7 pagesOrganic Chemistry Notemensahdelali73No ratings yet
- Alcohol OhDocument20 pagesAlcohol OhKara BarbosaNo ratings yet
- Alcohols Ethers and - 01-TheoryDocument59 pagesAlcohols Ethers and - 01-TheoryRaju SinghNo ratings yet
- Chapter16 UclassDocument88 pagesChapter16 Uclass배석우No ratings yet
- Ethers/Epoxides: Reactions With Brønsted Acids/BasesDocument13 pagesEthers/Epoxides: Reactions With Brønsted Acids/Baseslumik1234No ratings yet
- Science 9 Module 13Document28 pagesScience 9 Module 13DICES MNK Wesley L. ArconNo ratings yet
- Organic Chem NotesDocument21 pagesOrganic Chem NotesVeer PrajapatiNo ratings yet
- The Determination of Epoxide Groups: Monographs in Organic Functional Group AnalysisFrom EverandThe Determination of Epoxide Groups: Monographs in Organic Functional Group AnalysisNo ratings yet