Professional Documents
Culture Documents
Bonding, James Bonding
Uploaded by
Lachlan0 ratings0% found this document useful (0 votes)
3 views1 pageChem bonds
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentChem bonds
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views1 pageBonding, James Bonding
Uploaded by
LachlanChem bonds
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
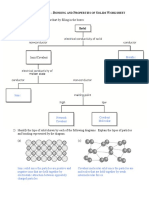
Metallic Bonding Ionic Bonding Covalent (Molecules) Covalent (Lattice)
Occurs Metal atoms Metal + Non-Metal Non-Metal + Non-metal Non-metal + Non-metal
Description Strong Electron(s) Sharing pair(s) of Electrons shared
ELECTROSTATIC transferred from electrons. Strong between every atom.
forces between Metal Non-metal electrostatic force of Strong electrostatic
METAL ION + Sea of Strong electrostatic attraction within forces (covalent bond)
DELOCALISED force called IONIC molecule called throughout entire
electrons BOND COVALENT BOND. sample.
Properties HIGH HIGH LOW HIGH
m.p/b.p
Electrical Good POOR POOR POOR
Conductivity Good GOOD POOR POOR
N/A GOOD POOR POOR
Solid Liquid
Solution
EXAMPLES Zn, Cu, any metal NaCl, MgSO4 H2O, NH3, CH4, O2 Diamond, silicon
dioxide (SiO2)
In order to conduct electricity a substance must possess
Delocalised electrons
OR
Mobile ions
You might also like
- Bonding TypesDocument18 pagesBonding TypesVed PatelNo ratings yet
- Chemical Bonding SummaryDocument8 pagesChemical Bonding SummaryKiara LimNo ratings yet
- Chapter 5Document3 pagesChapter 5s1062579No ratings yet
- Chemical Bonding & Molecular StructureDocument14 pagesChemical Bonding & Molecular StructureAYUSH GOSWAMINo ratings yet
- Midterm Chem86 NotesDocument9 pagesMidterm Chem86 NotessujzNo ratings yet
- Covalent Ionic: Forms MoleculesDocument1 pageCovalent Ionic: Forms Moleculesash100% (1)
- Bonding in Solids SummaryDocument2 pagesBonding in Solids SummaryarachnidkatNo ratings yet
- 1.3 Revision Guide Bonding AqaDocument3 pages1.3 Revision Guide Bonding AqaPragna AnanthNo ratings yet
- Chemical Bonds and Lewis StructuresDocument5 pagesChemical Bonds and Lewis Structuresnicole MenesNo ratings yet
- Bonding Iedxcel251Document13 pagesBonding Iedxcel251Best ProgressNo ratings yet
- 02 BondingDocument24 pages02 Bondingiron_trNo ratings yet
- Welcome To: Chemical Bonding and Molecular StructureDocument284 pagesWelcome To: Chemical Bonding and Molecular StructureSachin NayakNo ratings yet
- Comparison On BondsDocument7 pagesComparison On Bondseliastadele7No ratings yet
- (Lec4) Intermolecular and Intramolecular InteractionsDocument88 pages(Lec4) Intermolecular and Intramolecular InteractionsdinurjNo ratings yet
- Chemical Bonds and StructuresDocument8 pagesChemical Bonds and StructuresRainer VicencioNo ratings yet
- Chapter 1 Atomic BondingDocument23 pagesChapter 1 Atomic BondingLatisha AnthonyNo ratings yet
- Chemical Bonding and Molecular StructureDocument274 pagesChemical Bonding and Molecular StructureRohith KumarNo ratings yet
- 2 BondingDocument14 pages2 BondingRajasekar KrishnasamyNo ratings yet
- Lecture 5: Bonding Models: Ionic BondsDocument4 pagesLecture 5: Bonding Models: Ionic BondsmartinNo ratings yet
- Bonding A LevelDocument2 pagesBonding A LevelHamzah ArabicaNo ratings yet
- Electrochemistry: A Concise Overview of Key ConceptsDocument27 pagesElectrochemistry: A Concise Overview of Key Conceptskeycynarra.bonatrainologyNo ratings yet
- Lecture 1.4Document4 pagesLecture 1.4wemata7962No ratings yet
- 2 Atomic StructureDocument43 pages2 Atomic StructureRafael ArancibiaNo ratings yet
- L3 Fundamental Electrical PropertiesDocument52 pagesL3 Fundamental Electrical Propertieszwhmail1998No ratings yet
- Bonding and Structure-ReviewDocument1 pageBonding and Structure-Reviewcandyli3788No ratings yet
- Characterizing Ionic NetworksDocument3 pagesCharacterizing Ionic NetworksTrinh Tat-TranNo ratings yet
- Chemistry Test 5 Study GuideDocument3 pagesChemistry Test 5 Study GuideLeanne RoseNo ratings yet
- Chem Bonding Notes Ch7 2019Document2 pagesChem Bonding Notes Ch7 2019Kevin WeathersNo ratings yet
- Metals and Non-Metals React To Form Ionic CompoundsDocument2 pagesMetals and Non-Metals React To Form Ionic CompoundsDarshanaK 728714No ratings yet
- Chemical BondingDocument10 pagesChemical BondingseadiabaNo ratings yet
- 1 MetalsDocument39 pages1 MetalsManuel Tutacha ™No ratings yet
- Ionic V Covelant V MetallicDocument2 pagesIonic V Covelant V MetallicDesmondNo ratings yet
- 05 ANSWERS Summary of Structure & BondingDocument1 page05 ANSWERS Summary of Structure & BondingJean AlmiraNo ratings yet
- Property Explanation: Liquid StateDocument9 pagesProperty Explanation: Liquid StateNothing NameNo ratings yet
- Chapter 3 Chemical BondingDocument6 pagesChapter 3 Chemical BondingQutub KhanNo ratings yet
- Light and electron configurationDocument23 pagesLight and electron configurationHayley AndersonNo ratings yet
- Chemistry Nucleus-F: Theory Notes On Chemical Bonding-IDocument1 pageChemistry Nucleus-F: Theory Notes On Chemical Bonding-IRaju SinghNo ratings yet
- High School Chemistry - Core Concepts of Chemical BondingDocument1 pageHigh School Chemistry - Core Concepts of Chemical BondingDanilo Fronda Jr.No ratings yet
- 15: Chemical Bonding: Key Chemistry Terms Using Bond CharacteristicsDocument1 page15: Chemical Bonding: Key Chemistry Terms Using Bond Characteristicsbooty holeNo ratings yet
- Bonding and Properties of Solids Worksheet Solutions 1kadax6Document4 pagesBonding and Properties of Solids Worksheet Solutions 1kadax6Mel Patricia M. CabreraNo ratings yet
- Metallic BondingDocument22 pagesMetallic BondingnkjkjkjNo ratings yet
- Summary of Bonding, Structure and Properties of SubstancesDocument3 pagesSummary of Bonding, Structure and Properties of SubstancesAnonymous L7ZuSkR100% (1)
- Electrolytic Conductors and Electrolysis ReactionsDocument31 pagesElectrolytic Conductors and Electrolysis ReactionsSanchita Sarkar100% (1)
- Formation-Of-Ions and Chemical BondingDocument46 pagesFormation-Of-Ions and Chemical BondingMARY JOY PIOSCANo ratings yet
- Electrochemistry in 40 CharactersDocument60 pagesElectrochemistry in 40 CharactersSamarth GNo ratings yet
- How the Structure of Materials Affects Their PropertiesDocument15 pagesHow the Structure of Materials Affects Their PropertiesDITA FAUZI PRATAMANo ratings yet
- Ionic and Electronic DC Conduction - ElectrochemistryDocument27 pagesIonic and Electronic DC Conduction - ElectrochemistryWilliam Sin Chau WaiNo ratings yet
- Bonding Summary ChartDocument1 pageBonding Summary ChartКанат ТютеновNo ratings yet
- Bonding and Properties: Basics of Atomic StructureDocument13 pagesBonding and Properties: Basics of Atomic StructureAhsan AliNo ratings yet
- Covalent and Metallic Bonding: Test Yourself 7.1 (Page 114)Document2 pagesCovalent and Metallic Bonding: Test Yourself 7.1 (Page 114)khalil rehmanNo ratings yet
- Interatomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Document25 pagesInteratomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Anonymous BW2VsFifi9No ratings yet
- Covalent Network MoleculesDocument1 pageCovalent Network MoleculesGill CraigNo ratings yet
- Chemical Bonding LNDocument3 pagesChemical Bonding LNCenjie Niña Hayag SongcalNo ratings yet
- Core Chem Bonding Intro PresDocument43 pagesCore Chem Bonding Intro PresSHEILA MARIE CORTADO - UNDANNo ratings yet
- Paper 2Document21 pagesPaper 2John SonbolNo ratings yet
- 1 Bondintro Pres WebDocument19 pages1 Bondintro Pres Webbilal.ahmadNo ratings yet
- Interatomic Bonds: Prof. H. K. Khaira Hod, Msme Deptt. Manit, BhopalDocument81 pagesInteratomic Bonds: Prof. H. K. Khaira Hod, Msme Deptt. Manit, Bhopalraj kumarNo ratings yet
- Practice Worksheet of Chemical BondingDocument2 pagesPractice Worksheet of Chemical Bondingch khakanNo ratings yet
- Atomic Bonding in SolidsDocument24 pagesAtomic Bonding in Solidsazad832393No ratings yet
- Nero and Agrippina TranslDocument5 pagesNero and Agrippina TranslLachlanNo ratings yet
- Chapter 13Document1 pageChapter 13LachlanNo ratings yet
- Are We Controlling Science or Is Science Controlling Us?Document2 pagesAre We Controlling Science or Is Science Controlling Us?LachlanNo ratings yet
- How Does The Christian Worldview Influence The Lives of Adherents?Document3 pagesHow Does The Christian Worldview Influence The Lives of Adherents?LachlanNo ratings yet
- Yr 11 Catullus Translations and ComDocument19 pagesYr 11 Catullus Translations and ComLachlanNo ratings yet
- Intermolecular ForcesDocument1 pageIntermolecular ForcesLachlanNo ratings yet
- Tacitus Grammar Practice File 2Document6 pagesTacitus Grammar Practice File 2LachlanNo ratings yet
- 11 Latin Core HSC Vocab List For Assmt Part 2Document22 pages11 Latin Core HSC Vocab List For Assmt Part 2LachlanNo ratings yet
- Molecular ShapesDocument2 pagesMolecular ShapesLachlanNo ratings yet
- 1 Ignatian Person - Fred HollowsDocument3 pages1 Ignatian Person - Fred HollowsLachlanNo ratings yet
- Instrumental Genres of The Baroque PeriodDocument2 pagesInstrumental Genres of The Baroque PeriodLachlanNo ratings yet
- Ballade: Cantus Firmus: Church Modes: Conjunct Motion: Danse: ? Gregorian ChantDocument4 pagesBallade: Cantus Firmus: Church Modes: Conjunct Motion: Danse: ? Gregorian ChantLachlanNo ratings yet
- Baroque Music 1600 - 1750Document7 pagesBaroque Music 1600 - 1750LachlanNo ratings yet
- Music ConceptsDocument4 pagesMusic ConceptsLachlanNo ratings yet
- CantataDocument2 pagesCantataLachlanNo ratings yet
- Saint Ignatius' College Stage 5 - Pdhpe: Health Promotion StrategyDocument4 pagesSaint Ignatius' College Stage 5 - Pdhpe: Health Promotion StrategyLachlanNo ratings yet
- Extended Response EnglishDocument1 pageExtended Response EnglishLachlanNo ratings yet
- Year 10 Science Worksheet ReactionsDocument7 pagesYear 10 Science Worksheet ReactionsLachlanNo ratings yet
- Molecular ShapesDocument2 pagesMolecular ShapesLachlanNo ratings yet
- English (Advanced) Reading To WriteDocument1 pageEnglish (Advanced) Reading To WriteLachlanNo ratings yet
- Pedro Arrupe SJ - Leader of Jesuits Who Witnessed Hiroshima BombingDocument1 pagePedro Arrupe SJ - Leader of Jesuits Who Witnessed Hiroshima BombingLachlanNo ratings yet
- Okay CoolDocument1 pageOkay CoolLachlanNo ratings yet
- Refugee Writing PieceDocument1 pageRefugee Writing PieceLachlanNo ratings yet
- Year 10 Science Worksheet ReactionsDocument7 pagesYear 10 Science Worksheet ReactionsLachlanNo ratings yet