Professional Documents
Culture Documents
Chemistry Topic: Elements, Compounds and Mixtures
Uploaded by
amandaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry Topic: Elements, Compounds and Mixtures
Uploaded by
amandaCopyright:
Available Formats
Chemistry
Topic: Elements, Compounds and Mixtures
Introduction
-- Matter can be classified in terms of solid, liquid and gases. Matter can also be
classified in terms of element, compound or mixture.
Matter
1. Pure substance
Element
o Metal
o Non-metal
o Metalloids
Compound
2. Mixture
Element + Element
Element + Compound
Compound + Compound
*An element or a compound, which contains a small amount of impurity (i.e. another
element or compoun), is considered as an impure substance. Thus a mixture can also
be regarded as an impure substance.
Element
-- Pure substance that cannot be split up into two or more simpler substances by
chemial processes or by electricity.
-- Classified into metals, metalloids and non-metals. (Most elements are metal)
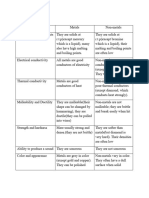
Properties Metals Non-metals
Appearance Shiny Dull if solid
State Solids at r.t.p (Except for mecury) Either gases, volatile liquids or
solids with low melting points at
r.t.p. (Except for carbon)
Malleable Can be hammered into different Brittle if solid (Easily broken
shapes without breaking when hammered)
Sonorous Make a ringing sound when
struck
Ductile (able Can be drawn into wires
to be
deformed)
Melting & High melting and boiling points Low melting and boiling points
Boiling (Except for sodium, potassium (Except for carbon and silicon)
Points and mercury)
Thermal Good conductors of heat Poor conductors of heat (Except
Conductivity carbon in the form of diamond
and graphite)
Electrical Good conductors of electricity in Poor conductors of electricity
Conductivity all states of matter (Except carbon in the form of
graphite)
You might also like
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- Ch5.1 Classification of Elements (Summary)Document2 pagesCh5.1 Classification of Elements (Summary)Tony 852No ratings yet
- Chemistry 7A: Atoms and Elements: Sean, Davin, Valen, Mathew, CatherineDocument5 pagesChemistry 7A: Atoms and Elements: Sean, Davin, Valen, Mathew, Catherinesean gillmoreNo ratings yet
- Summary Notes - YR11 Module 1 (Chemistry)Document16 pagesSummary Notes - YR11 Module 1 (Chemistry)tcs517090No ratings yet
- Form 1 Science Chapter 6Document10 pagesForm 1 Science Chapter 6Koo SengleeNo ratings yet
- Metals and Non MetalDocument1 pageMetals and Non MetalHanehs NoLsubNo ratings yet
- Uydz Uw WV USKa N61 MM JC 4Document6 pagesUydz Uw WV USKa N61 MM JC 4varshatagade126No ratings yet
- Geography Lesson 2Document5 pagesGeography Lesson 2indaneNo ratings yet
- Grade X - Teaching Notes: Metals and Non-Metals GlossaryDocument25 pagesGrade X - Teaching Notes: Metals and Non-Metals GlossaryAkshith KottaNo ratings yet
- Chapter - 3 Metals - and - Non - Metals NewDocument14 pagesChapter - 3 Metals - and - Non - Metals NewRahul IngleNo ratings yet
- Atomic Structure and PeriodicityDocument9 pagesAtomic Structure and PeriodicityYash BhattNo ratings yet
- Metals and Non-MetalsDocument30 pagesMetals and Non-MetalsAyushi SinghNo ratings yet
- Properties of MetalsDocument4 pagesProperties of MetalsYanika BarasNo ratings yet
- 6 The Periodic Table (Teacher)Document42 pages6 The Periodic Table (Teacher)otto wongNo ratings yet
- Metals and Non Metals Notes Class 10 2022-23Document37 pagesMetals and Non Metals Notes Class 10 2022-23ramkumarsingh12406100% (1)
- CBSE Class 10 Science Notes Chapter 3 2Document8 pagesCBSE Class 10 Science Notes Chapter 3 2Maithili PanwarNo ratings yet
- Groups in The Periodic Table of ElementsDocument7 pagesGroups in The Periodic Table of ElementsBRYAN bryan MacadangdangNo ratings yet
- Classification of MatterDocument17 pagesClassification of MatterAshmyra ManaloNo ratings yet
- Properties of MetalsDocument4 pagesProperties of MetalsKevin Joe CuraNo ratings yet
- PWSUNP223620230816131206848PPT - Metals, Nonmetals, MetalloidsDocument18 pagesPWSUNP223620230816131206848PPT - Metals, Nonmetals, Metalloidsrishit.yadav1102No ratings yet
- Comparison of Physical and Chemical Properties of Metals and Non - MetalsDocument3 pagesComparison of Physical and Chemical Properties of Metals and Non - MetalsSWATINo ratings yet
- Metals and Non MetalsDocument23 pagesMetals and Non Metalsshaunchinu patilNo ratings yet
- Non-Metallic ElementsDocument6 pagesNon-Metallic ElementsAla'No ratings yet
- KSSM F1 C6Document74 pagesKSSM F1 C6Tanisa SaminNo ratings yet
- Metals and Non-Metals: Year 9 Christmas Term Week 1Document11 pagesMetals and Non-Metals: Year 9 Christmas Term Week 1OlajumokeNo ratings yet
- Metals & Non-Metals: Topics in The ChapterDocument7 pagesMetals & Non-Metals: Topics in The ChapterManya SinghviNo ratings yet
- Metals & Non MetalsDocument2 pagesMetals & Non MetalsLakshminarayanaNo ratings yet
- Metals and Non-MetalsDocument20 pagesMetals and Non-MetalsDevyansh MishraNo ratings yet
- Metals and Non-MetalsDocument11 pagesMetals and Non-MetalsRoty005100% (4)
- Element Classification of Elements: Class-X Chapter - 3 Metals and Non-MetalsDocument11 pagesElement Classification of Elements: Class-X Chapter - 3 Metals and Non-MetalsInsha Hasan 10DNo ratings yet
- Chapter 2 - Is Matter Around Us Pure - Summary Note: Sub-TopicsDocument14 pagesChapter 2 - Is Matter Around Us Pure - Summary Note: Sub-TopicsRanjeet SinghNo ratings yet
- AssignmentDocument13 pagesAssignmentfarusadiq.77No ratings yet
- Metals and Non-MetalsDocument10 pagesMetals and Non-MetalsCherry IkemNo ratings yet
- Metals and NonmetalsDocument2 pagesMetals and NonmetalsAnnisa Ekaputri FebrianiNo ratings yet
- Chapter - 3 Metals and Non - Metals Gist of The LessonDocument8 pagesChapter - 3 Metals and Non - Metals Gist of The LessonPrasadNo ratings yet
- Chapter 12 - The Periodic Table PDFDocument4 pagesChapter 12 - The Periodic Table PDFAarush SharmaNo ratings yet
- IGCSE Chemistry - Unit 12 - The Periodic TableDocument6 pagesIGCSE Chemistry - Unit 12 - The Periodic TableRaffaella LaxaldeNo ratings yet
- 2324 T2 Chemistry C3 Elements and CompoundsDocument66 pages2324 T2 Chemistry C3 Elements and CompoundswilsonconcepcionNo ratings yet
- Materials Metals and Non-MetalsDocument5 pagesMaterials Metals and Non-Metalssidhantm823No ratings yet
- Metals and Non MetalsDocument29 pagesMetals and Non Metalsayushmallick07No ratings yet
- Metals and Non MetalsDocument23 pagesMetals and Non Metalsreshma1917singhNo ratings yet
- Sains Chpter 6Document72 pagesSains Chpter 6hidayahsanusi tft5001No ratings yet
- Period IcDocument72 pagesPeriod Icmasterupsi2014No ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- Revision Notes On Materials Metals and Non-MetalsDocument9 pagesRevision Notes On Materials Metals and Non-MetalsHoang HaNo ratings yet
- IGCSE Edexcel Chemistry - Chapter 10, 11, 12, 14, 15Document4 pagesIGCSE Edexcel Chemistry - Chapter 10, 11, 12, 14, 15JoannaNo ratings yet
- Metals and Non Metals - NotesDocument8 pagesMetals and Non Metals - NotesMohita RastogiNo ratings yet
- 9E Reactions of Metals andDocument18 pages9E Reactions of Metals and陳信羽No ratings yet
- Reading Material by NVS TeacherDocument12 pagesReading Material by NVS Teacher10E Yuvan Sarabeshan Thirumeninathan [3383]No ratings yet
- Science: (Chemistry)Document22 pagesScience: (Chemistry)Isaiah JohnsonNo ratings yet
- CH 3Document32 pagesCH 3Prachi RaniNo ratings yet
- Metals and Non MetalsDocument2 pagesMetals and Non MetalsNavkiran Ladhar 279No ratings yet
- Metals and Non-MetalsDocument25 pagesMetals and Non-MetalsXun Rou ChamNo ratings yet
- PharChem Lecture Reviewer Part IDocument5 pagesPharChem Lecture Reviewer Part ICarmelle Zia ReyesNo ratings yet
- 9 Science Metals NonmetalsDocument7 pages9 Science Metals NonmetalsAjay AnandNo ratings yet
- Metals and NonmetalsDocument2 pagesMetals and NonmetalsSenthil Kumar PNo ratings yet
- Metalic Elements and PropertiesDocument5 pagesMetalic Elements and PropertiesM.zuhair asifNo ratings yet
- Class 10 Metals and Non Metals NotesDocument12 pagesClass 10 Metals and Non Metals NotesShreyash VishwakarmaNo ratings yet
- Metallic and Nonmetallic PropertiesDocument24 pagesMetallic and Nonmetallic PropertiesEleanor Alma JuguetaNo ratings yet
- JSA - Metals Vs Non MetalsDocument23 pagesJSA - Metals Vs Non Metalsj.sandNo ratings yet