Professional Documents
Culture Documents
Biopharma Ar Broschuere Englisch
Uploaded by
azmatCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Biopharma Ar Broschuere Englisch
Uploaded by
azmatCopyright:
Available Formats
An interactive journey
through biopharmacy
This brochure contains interactive augmented
reality 3D content – accessible with the free
app Boehringer Ingelheim AR (iOS/Android).
105x148_BO Booklet 2019_EN.indd 1 15.03.2019 08:33:01
2
This brochure contains augmented reality content.
Scan this QR code and download
the free app Boehringer
Ingelheim AR from the Apple
App store/iTunes (iOS) or the
Google Play store (Android).
Boehringer Ingelheim BIOPHARMACY V ienna 2019
105x148_BO Booklet 2019_EN.indd 2 15.03.2019 08:33:03
3
After installing the app, focus your camera on
the images in the brochure to bring interactive
augmented reality 3D content to life, and to access
lots of additional information about development
and production at Boehringer Ingelheim in Vienna.
Boehringer Ingelheim BIOPHARMACY V ienna 2019
105x148_BO Booklet 2019_EN.indd 3 15.03.2019 08:33:05
4
Welcome to the
Boehringer Ingelheim
World of Biopharmacy!
Pioneer and Top Provider
in the Biopharmaceutical Sector
Dr. Christian Eckermann
Head of Biopharma Austria
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 4 15.03.2019 08:33:09
5
We produce high-quality pharmaceuticals
(biopharmaceuticals) with the help of living
cells. This enables us to offer therapies to
people around the world for diseases such
as multiple sclerosis and osteoporosis, or to
support blood thinning.
Would you like to know how many
atoms make up our largest active
substance molecule?
BIanca will tell you, if you scan the
image, using your mobile phone.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 5 15.03.2019 08:33:12
6
The campus of Boehringer Ingelheim
in Austria
All the functions required to produce a
biopharmaceutical drug can be found at our
site in Vienna’s Meidling district – one of the
four global biopharmaceutical sites in the
group of companies.
In addition to biopharmaceuticals, it is also the
hub for the human pharmaceuticals and animal
health business for more than 30 countries and
Boehringer Ingelheim’s main cancer research
center.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 6 15.03.2019 08:33:16
7
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 7 15.03.2019 08:33:31
8
Teamwork is required when we
develop a new drug. Specialists
from development, production,
quality and many other fields work
together using state-of-the-art
technology to help patients around
the world.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 8 15.03.2019 08:33:32
9
We also produce on behalf of other pharmaceutical
companies and are the world market leader!
We have launched a total of 31 biopharmaceuticals
onto the global market for ourselves and our
customers.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 9 15.03.2019 08:33:33
10
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 10 15.03.2019 08:33:38
11
The development and optimization of
the production process starts with a few
milliliters, which can be increased up to
15,000 liters in our large-scale plants, to
produce different active ingredients and
quantities. Flexibility and experience are
our greatest strengths.
In our largest fermenter (vessel for cell
proliferation) on site, we can produce up to one
quadrillion (a number with 15 zeros) cells.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 11 15.03.2019 08:33:43
12
In biopharmacy, the focus is on perfection.
From the raw materials to the end products,
everything is carefully checked, analyzed and
documented, enabling us to ensure quality in
every phase of the process chain.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 12 15.03.2019 08:33:45
13
Every year, we are subject to
approximately twelve inspections by
health authorities and customers from
all over the world, who inspect our
plants and operations very carefully.
This means an average of once a month!
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 13 15.03.2019 08:33:46
14
Automated systems help us throughout the
entire development and production process
to manage data, generate visualizations and
help control the facilities.
Digitalisation also enables different systems
to communicate with each other and to
reduce manual interventions to a minimum.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 14 15.03.2019 08:33:48
15
Our cell culture facility is equipped with a
fully digitized operation concept. The data
from approximately 20,000 metering points
are continuously available during the entire
production process.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 15 15.03.2019 08:33:50
16
Boehringer Ingelheim maintains close
cooperations with universities,
colleges, start-up companies
and non-university research
institutions at home and
abroad with the aim of
always using state-of-
the-art technologies.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 16 15.03.2019 08:33:53
17
We work together with 15 of
the top 20 pharmaceutical
companies worldwide.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 17 15.03.2019 08:33:56
18
The Vienna location is developing on an
ongoing basis. In April 2017, a further milestone
was reached with the ground-breaking
ceremony for a cell culture plant.
The total investment of about 700 million euros
represents the largest single investment in over
130 years of company history, enabling us to
create 500 new jobs at the Vienna location.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 18 15.03.2019 08:33:57
19
Vienna is the only location in the group of
companies where human pharmaceuticals are
produced both on the basis of microorganisms
(bacteria, yeasts) and with the aid of
mammalian cells.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 19 15.03.2019 08:33:57
20
Boehringer Ingelheim is a family-owned
company that lives up to its social responsibility.
Therefore it is important for us to create a good
working atmosphere. Whether it is a company
day care facility, sports facilities or an employee
restaurant – we offer a variety of benefits, a
collaborative environment and a good work/life
balance.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 20 15.03.2019 08:33:58
21
Continuous professional development is also a
key topic. Theoretical and practical content can
be perfectly adapted to the job in a real world
environment thanks to our own training facility.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 21 15.03.2019 08:34:00
22
How long has Boehringer Ingelheim Vienna been doing
research in the field of biotechnology?
D) over 70 years N) over 30 years
C) over 50 years I) over 10 years
Which cells at Boehringer Ingelheim are not used to
produce drugs?
G) bacteria F) yeast
R) mammalian cells E) insect cells
What happens during the fermentation of a
biopharmaceutical drug?
L) cell proliferation and formation of the active ingredient
B) alcohol is produced
K) oxygen is formed
S) tablets are produced
How many new biopharmaceutical employees will be
taken on in Vienna by 2021?
A) 200 L) 500
U) 400 P) 700
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 22 15.03.2019 08:34:02
23
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 23 15.03.2019 08:34:04
24
Environmental protection, health, safety and
responsible business conduct are part of our DNA.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 24 15.03.2019 08:34:07
25
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 25 15.03.2019 08:34:11
26
Our thanks go to all those who have
supported us on our way so far – we
look forward to continuing our success
together in the future.
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 26 15.03.2019 08:34:15
27
Legal notice:
Media owner, editor, publisher:
Boehringer Ingelheim RCV GmbH & Co KG,
A-1121 Vienna, Dr. Boehringer-Gasse 5-11,
Phone: + 43 (1) 801 05-0
Responsible for the content: Dr. Christian Eckermann
Editorial: Cornelia Wieser
Layout: Belinda Estl
Proofreader: Anneliese Krabina-Lindner
Translation: ALL Languages Alice Rabl GmbH, Wien
App programming: Martin Chiettini, Bobby Rajesh Malhotra,
Sebastian Pirch
Photographs: Adobe Stock, MirAlf Filmproduktion,
Rainer Mirau, Hubert Weiser
Campus map: Quadratmeter OEG
Visualisation virtual-reality-model production building:
©Architekturbüro Wolfgang Wildauer
Music clip: https://audiojungle.net/item/inspiring/9325839?s_rank=1
Speakers: Nicola Gold, Anneliese Krabina-Lindner
Motion Capturing: Anneliese Krabina-Lindner
CFD visualisation: Johannes Wutz
Copyright
© All rights reserved, in particular the right of reproduction (in whole
or in part). The brochure may not be reproduced or duplicated using
electronic systems without the written permission of Boehringer
Ingelheim RCV GmbH & Co KG.
January 2019
Website: www.boehringer-ingelheim.at
Job page: https://careers.boehringer-ingelheim.com/
Facebook: https://www.facebook.com/BoehringerAT
BioXcellence: https://www.bioxcellence.com/
Making More Health: www.makingmorehealth.org
Boehringer Ingelheim BIOPHARMACY VIENNA 2019
105x148_BO Booklet 2019_EN.indd 27 15.03.2019 08:34:19
Boehringer Ingelheim RCV GmbH & Co KG
Dr. Boehringer-Gasse 5-11
A-1121 Vienna
Phone: + 43 (1) 801 05-0
Fax: + 43 (1) 804 08 23
www.boehringer-ingelheim.com
105x148_BO Booklet 2019_EN.indd 28 15.03.2019 08:34:19
You might also like
- Formal Company ProfileDocument2 pagesFormal Company ProfileMvk KhumaloNo ratings yet
- Social Media PlanDocument10 pagesSocial Media PlanShelby MustainNo ratings yet
- Drug Discovery and Biotechnolgy in GermanyDocument77 pagesDrug Discovery and Biotechnolgy in GermanyVenkata Suryanarayana GorleNo ratings yet
- Press Information: White BiotechnologyDocument3 pagesPress Information: White BiotechnologysugaremilioNo ratings yet
- Why Dont Our Vaccines Reach The Highhanging FruitDocument1 pageWhy Dont Our Vaccines Reach The Highhanging FruitAnil MelwaniNo ratings yet
- Boehringer Ingelheim Congratulates Nobel Prize Winner ProfDocument1 pageBoehringer Ingelheim Congratulates Nobel Prize Winner ProfSunny YadavNo ratings yet
- JurnalDocument14 pagesJurnalWindi Sari PattyNo ratings yet
- Purell GelPads - Marketing PlanDocument45 pagesPurell GelPads - Marketing PlanRidhima KalraNo ratings yet
- QC Microbiology AnalystDocument1 pageQC Microbiology AnalystTanuwijaya Jaya SugiNo ratings yet
- Inaugural Speech - Introduction and Welcome: (Intro)Document4 pagesInaugural Speech - Introduction and Welcome: (Intro)Estari MamidalaNo ratings yet
- Lupin and Boehringer Ingelheim Announce Strategic Co-Marketing Agreement (Company Update)Document2 pagesLupin and Boehringer Ingelheim Announce Strategic Co-Marketing Agreement (Company Update)Shyam SunderNo ratings yet
- AD-Times - Boehringer IngelheimDocument12 pagesAD-Times - Boehringer IngelheimAkash GoyalNo ratings yet
- Private Labels For Premium Products - The Example of Organic FoodDocument18 pagesPrivate Labels For Premium Products - The Example of Organic FoodajeetkharraNo ratings yet
- Manstra - Final Strategy PaperDocument18 pagesManstra - Final Strategy PaperMARIA CRISTINA DE PAZNo ratings yet
- Report Immunite Marketing PlanDocument15 pagesReport Immunite Marketing PlanmanavNo ratings yet
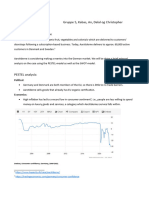
- Content: 2.1: PESTEL 2.2: SWOT Analysis 2.3: Michael Porter's Five ForcesDocument7 pagesContent: 2.1: PESTEL 2.2: SWOT Analysis 2.3: Michael Porter's Five ForcesAhmed KamalNo ratings yet
- Content: 2.1: PESTEL 2.2: SWOT Analysis 2.3: Michael Porter's Five ForcesDocument7 pagesContent: 2.1: PESTEL 2.2: SWOT Analysis 2.3: Michael Porter's Five ForcesAhmed KamalNo ratings yet
- Case 1 Årstiderne EksternalDocument3 pagesCase 1 Årstiderne EksternalKabas Karim NashiNo ratings yet
- Biomass Protein - Sven ThormählenDocument17 pagesBiomass Protein - Sven Thormählenjuanpineda64No ratings yet
- BSTR565Document16 pagesBSTR565Prerna SinghNo ratings yet
- AR19 Final Buhler Annual Report 2019Document147 pagesAR19 Final Buhler Annual Report 2019Phương TrầnNo ratings yet
- Food Fraud Prevention PDFDocument80 pagesFood Fraud Prevention PDFRAFAEL SOLANONo ratings yet
- Press Release: Box 8861 405 31 Gothenburg Sweden Telephone: +46 (0) 10 335 00 00Document3 pagesPress Release: Box 8861 405 31 Gothenburg Sweden Telephone: +46 (0) 10 335 00 00bobNo ratings yet
- Pharma Emarketing Europe 2011Document8 pagesPharma Emarketing Europe 2011Eye For Pharma EventsNo ratings yet
- 22 ZanderDocument11 pages22 ZanderCharlieNo ratings yet
- Vitafoods Europe 2019 New Exhibitor DirectoryDocument12 pagesVitafoods Europe 2019 New Exhibitor Directoryttquyen04No ratings yet
- Given Imaging: Team 2Document7 pagesGiven Imaging: Team 2Anirban PalNo ratings yet
- The Roadmap Report: Unit 2: The Return of The Milkman Corresponds With Lesson 2ADocument2 pagesThe Roadmap Report: Unit 2: The Return of The Milkman Corresponds With Lesson 2ArunnermnNo ratings yet
- Biocon Annual Meeting 200705Document4 pagesBiocon Annual Meeting 200705himarc76No ratings yet
- News Release: Bayer With Continued Strong PerformanceDocument7 pagesNews Release: Bayer With Continued Strong PerformancePRAKASHNo ratings yet
- GroupExercise1 InstructionsDocument7 pagesGroupExercise1 InstructionsmarceloraboNo ratings yet
- Pharmacy Daily For Thu 09 Aug 2012 - Telehealth Future, Expanded Prolia, BioCeuticals Upgrade, Nurofen Controversy and Much More...Document2 pagesPharmacy Daily For Thu 09 Aug 2012 - Telehealth Future, Expanded Prolia, BioCeuticals Upgrade, Nurofen Controversy and Much More...pharmacydailyNo ratings yet
- Ophirtech CPDocument21 pagesOphirtech CPJexter MorojoNo ratings yet
- Porter Five Force Model of ENGRO 1) Threat of New EntrantsDocument3 pagesPorter Five Force Model of ENGRO 1) Threat of New Entrantskhalidhussainraza100% (1)
- Bayer R1Document8 pagesBayer R1DáshingBôyNo ratings yet
- Industrial BiotechnologyDocument9 pagesIndustrial BiotechnologyRohailNo ratings yet
- Perstorp New Buffering TecnologyDocument1 pagePerstorp New Buffering TecnologyHéctor M RavennaNo ratings yet
- IFPMA Facts and Figures 2021Document102 pagesIFPMA Facts and Figures 2021sreedhardgNo ratings yet
- Consumer Behaviour Towards Organic Products: The Moderating Role of Environmental ConcernDocument13 pagesConsumer Behaviour Towards Organic Products: The Moderating Role of Environmental ConcernNilesh HosureNo ratings yet
- Bulletin of The IDF #502 - 2019 - Ecology of Listeria Spp. and Listeria Monocytogenes - CAT PDFDocument52 pagesBulletin of The IDF #502 - 2019 - Ecology of Listeria Spp. and Listeria Monocytogenes - CAT PDFAriel VicenteNo ratings yet
- Quality Control Analysis To Press The Finished Goods Damage Quantity at PT - Boehringer Ingelheim IndonesiaDocument14 pagesQuality Control Analysis To Press The Finished Goods Damage Quantity at PT - Boehringer Ingelheim IndonesiaNur fadilah febrianiNo ratings yet
- Role of IP in BiotechDocument12 pagesRole of IP in BiotechLalit AmbasthaNo ratings yet
- Shareholder's ActivismDocument2 pagesShareholder's ActivismAlice dlbNo ratings yet
- Biotechnology:: Personal Care Market ReportDocument18 pagesBiotechnology:: Personal Care Market ReportjoseNo ratings yet
- NIBS Business Plan Competition Sample 3 PDFDocument15 pagesNIBS Business Plan Competition Sample 3 PDFanika bhuianNo ratings yet
- Pi9257 7632 En-UsDocument2 pagesPi9257 7632 En-Usخبراء التصنيع الدوائي-اليمنNo ratings yet
- Bayer - Reestruct Organizativa 29.11.18Document6 pagesBayer - Reestruct Organizativa 29.11.18Kevin CornejoNo ratings yet
- Task 42 Biobased Chemicals - Value Added Products From Biorefineries PDFDocument36 pagesTask 42 Biobased Chemicals - Value Added Products From Biorefineries PDFeliasiqNo ratings yet
- Biocon Annual Report 2015Document216 pagesBiocon Annual Report 2015Rajeev RsNo ratings yet
- Competitors AnalysisDocument12 pagesCompetitors AnalysisDaniyal khanNo ratings yet
- Does A Brand Need A Purpose?Document10 pagesDoes A Brand Need A Purpose?scarlettsmlauNo ratings yet
- Biocon Eyes RDocument3 pagesBiocon Eyes RragipanidineshNo ratings yet
- A Business Plan For Poultry FarmDocument8 pagesA Business Plan For Poultry FarmArjel MeregildoNo ratings yet
- Feasibility StudyDocument47 pagesFeasibility StudyCyril Fragata100% (1)
- Swot Analysis of Maandeeq CompanyDocument2 pagesSwot Analysis of Maandeeq CompanyNasrudiinNo ratings yet
- Ecp Proj Ref2Document102 pagesEcp Proj Ref2tanyaNo ratings yet
- Project Plant Based MeatDocument54 pagesProject Plant Based MeatVijay Chandra MNo ratings yet
- Cover LatterDocument1 pageCover LatterDattatray DoifodeNo ratings yet
- Biogas: Introduction, Recycling, Systems and Technology, Recycling of Residues, Information PlatformsDocument37 pagesBiogas: Introduction, Recycling, Systems and Technology, Recycling of Residues, Information PlatformsazmatNo ratings yet
- 4 AzDocument63 pages4 AzazmatNo ratings yet
- Measurement, Regulation and AutomationDocument77 pagesMeasurement, Regulation and AutomationazmatNo ratings yet
- 2 1.de - enDocument64 pages2 1.de - enazmatNo ratings yet
- Biogas Generation & Algae Biotechnology: SubjectsDocument4 pagesBiogas Generation & Algae Biotechnology: SubjectsazmatNo ratings yet
- 2 1.de - enDocument64 pages2 1.de - enazmatNo ratings yet
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationIOSR Journal of Pharmacy100% (1)
- PDB SequencesDocument4 pagesPDB SequencesazmatNo ratings yet
- DBFZ Report 33Document120 pagesDBFZ Report 33azmatNo ratings yet
- Leu TDocument24 pagesLeu TazmatNo ratings yet
- Andersen-2009-Location of The Antidepressant 1Document9 pagesAndersen-2009-Location of The Antidepressant 1azmatNo ratings yet
- Life Is HorrorDocument1 pageLife Is HorrorazmatNo ratings yet
- Bioenergy: Renewable Energy in Austria Energy From BiomassDocument10 pagesBioenergy: Renewable Energy in Austria Energy From BiomassazmatNo ratings yet
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationIOSR Journal of Pharmacy100% (1)
- Brummel Hu Is 1985Document10 pagesBrummel Hu Is 1985azmatNo ratings yet
- Mary Hloomcraft: Work ExperienceDocument2 pagesMary Hloomcraft: Work ExperienceazmatNo ratings yet
- GMPDocument19 pagesGMPSoad Nafi100% (1)
- Life in Austria in EnglishDocument23 pagesLife in Austria in EnglishazmatNo ratings yet
- Be Be Be Be Be Be Be: Cor Res Pon Ds Something Is Taken Away Verb Fat, WeighDocument4 pagesBe Be Be Be Be Be Be: Cor Res Pon Ds Something Is Taken Away Verb Fat, WeighazmatNo ratings yet
- Harry - Gefangen in Der Zeit: BegleitmaterialienDocument7 pagesHarry - Gefangen in Der Zeit: BegleitmaterialienazmatNo ratings yet
- Life Is A FunDocument1 pageLife Is A FunazmatNo ratings yet
- Modelling MethodsDocument1 pageModelling MethodsazmatNo ratings yet
- File Is ReadyDocument1 pageFile Is ReadyazmatNo ratings yet
- Harry Folge001 100Document695 pagesHarry Folge001 100azmat100% (1)
- Journal Pone 0005009 PDFDocument4 pagesJournal Pone 0005009 PDFazmatNo ratings yet
- File Is ReadyDocument1 pageFile Is ReadyazmatNo ratings yet
- File Is ReadyDocument1 pageFile Is ReadyazmatNo ratings yet
- File Is ReadyDocument1 pageFile Is ReadyazmatNo ratings yet
- The Mystique of The Dominant WomanDocument8 pagesThe Mystique of The Dominant WomanDorothy HaydenNo ratings yet
- Final PR 2 CheckedDocument23 pagesFinal PR 2 CheckedCindy PalenNo ratings yet
- Inversor Abb 3 8kwDocument2 pagesInversor Abb 3 8kwapi-290643326No ratings yet
- Expressions of QuantityDocument5 pagesExpressions of Quantitybenilde bastidaNo ratings yet
- Circulatory SystemDocument51 pagesCirculatory SystemTina TalmadgeNo ratings yet
- Theoretical Background: Theories Relevance To The Study SourcesDocument3 pagesTheoretical Background: Theories Relevance To The Study SourcesAdelfa Mae BerdonNo ratings yet
- Ems em FW Paneel Firetec enDocument2 pagesEms em FW Paneel Firetec enzlatkokrsicNo ratings yet
- Summative Test SolutionsDocument1 pageSummative Test SolutionsMarian Anion-GauranoNo ratings yet
- Life Orientation Grade 11 Revision Term 2 - 2021 FinalDocument16 pagesLife Orientation Grade 11 Revision Term 2 - 2021 FinalTeeshan VerappenNo ratings yet
- Inside The Earth NotesDocument2 pagesInside The Earth NotesrickaturnerNo ratings yet
- SpectraSensors TDL Analyzers in RefineriesDocument8 pagesSpectraSensors TDL Analyzers in Refineries1977specopsNo ratings yet
- Deductions From Gross IncomeDocument2 pagesDeductions From Gross Incomericamae saladagaNo ratings yet
- Physical Activity and Weight ControlDocument6 pagesPhysical Activity and Weight Controlapi-288926491No ratings yet
- Physio Essay #4Document2 pagesPhysio Essay #4Maria Margarita Chon100% (1)
- Melancholic PersonalityDocument5 pagesMelancholic PersonalityChris100% (1)
- 168 Visual Perceptual SkillsDocument3 pages168 Visual Perceptual Skillskonna4539No ratings yet
- Sialoree BotoxDocument5 pagesSialoree BotoxJocul DivinNo ratings yet
- 2012 U.S. History End-of-Course (EOC) Assessment Field Test Fact SheetDocument2 pages2012 U.S. History End-of-Course (EOC) Assessment Field Test Fact SheetswainanjanNo ratings yet
- Powador 7700 - 7900 8600 - 9600: OriginalDocument52 pagesPowador 7700 - 7900 8600 - 9600: Originalashraf-84No ratings yet
- QRG-DC-004 Procedure and Regulation Governing The Requirements For CPWDocument56 pagesQRG-DC-004 Procedure and Regulation Governing The Requirements For CPWKarthi Keyan100% (2)
- Hotel ClassificationDocument10 pagesHotel ClassificationRonelyn Boholst100% (1)
- Unsaturated HydrocarbonsDocument84 pagesUnsaturated HydrocarbonsHey itsJamNo ratings yet
- Module 5 The Teacher and The Community School Culture and Organizational LeadershipDocument6 pagesModule 5 The Teacher and The Community School Culture and Organizational LeadershipHazeldiazasenas100% (6)
- Electrolux EKF7700 Coffee MachineDocument76 pagesElectrolux EKF7700 Coffee MachineTudor Sergiu AndreiNo ratings yet
- Group Interative Art TherapyDocument225 pagesGroup Interative Art TherapyRibeiro CatarinaNo ratings yet
- Electronic Fetal MonitoringDocument4 pagesElectronic Fetal MonitoringMauZungNo ratings yet
- Hubungan Faktor Lokal, Faktor Sistemik Dan Faktor Perilaku Terhadap Kejadian Penyakit Periodontal Di Indonesia (Analisis Riskesdas)Document10 pagesHubungan Faktor Lokal, Faktor Sistemik Dan Faktor Perilaku Terhadap Kejadian Penyakit Periodontal Di Indonesia (Analisis Riskesdas)lidyaNo ratings yet
- NIST Standard Reference Materials® 2023 CatalogDocument128 pagesNIST Standard Reference Materials® 2023 CatalogAbdul HaseebNo ratings yet
- Saa6d107e 1CC S N 26540705 Up - Parts Book Do Motor GD655-5Document164 pagesSaa6d107e 1CC S N 26540705 Up - Parts Book Do Motor GD655-5kit101No ratings yet
- NHT Series High-Throughput Diffusion PumpsDocument12 pagesNHT Series High-Throughput Diffusion PumpsJosé Mauricio Bonilla TobónNo ratings yet