Professional Documents
Culture Documents
Standardization of Acid-Base Titration
Uploaded by
Samantha Jesi Shania EstradaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Standardization of Acid-Base Titration
Uploaded by
Samantha Jesi Shania EstradaCopyright:
Available Formats
Samantha Jesi Shania D.
Estrada Date Submitted: 10/02/2020
BioSc 1B
STANDARDIZATION OF ACID-BASE TITRATION
INTRODUCTION
Titration is the process of measuring the volume of one reagent required to react with a measured
volume or weight of another reagent. The goal is to stop the addition of reagent when stoichiometrically
equal amounts of the two reactants have been combined. Standardization is the process of determining
the exact concentration (molarity) of a solution. The reaction of an acid and a base to form a salt and water
is known as neutralization. This experiment has 3 parts: (A) Preparation and Standardization of Sodium
Hydroxide, (B) Analysis of Unknown KHP, and (C) Analysis of Unknown Acid.
OBJECTIVES
1. To standardize the Sodium hydroxide solution with Potassium hydrogen phthalate (KHP).
2. To determine the percent mass of KHP in an unknown mixture.
3. To determine the Molar Mass of an Unknown Monoprotic/Diprotic/Triprotic Acid.
METHODOLOGY
Preparation of 1 L carbonate –free 0.1 M NaOH Solution
1.) Boil approximately 2 L of distilled water in a beaker for at least 15 minutes. To minimize bumping,
place a stirring rod inside the beaker and cover it with a watch glass
2.) While waiting for the solution to boil, look for two (2) 1 liter polyethylene containers for the
solution. Wash it with soap and water.
3.) Calculate the amount of NaOH pellets needed to prepare 1 liter of 0.1 M NaOH solution.
4.) When the distilled water is near boiling, weigh this amount on a watch glass using a top-loading
balance. Warning: Sodium hydroxide pellets is corrosive, avoid skin contact with this chemical. Wear
latex gloves while weighing NaOH pellets. Wipe out chemical spills on the balance because this will
corrode the equipment.
5.) Transfer the NaOH pellets in a 1 liter beaker. Dissolve the NaOH pellets with approximately 500 ml
of carbonate free distilled water, then dilute the solution to the 1 liter mark.
6.) Rinse the container with a small amount of the prepared solution.
7.) Place the container in an ice-water bath and transfer the prepared solution quantitatively into the
clean bottle. Cover the bottle tightly.
8.) Let the solution cool down to room temperature in the ice water.
9.) Label the container; write your name and the date the solution was prepared.
10.) Keep the extra carbonate free distilled water in another clean polyethylene bottle.
Standardization of the prepared NaOH Solution
1.) Heat the primary standard - potassium hydrogen phthalate (KHP), KHC8H4O4 in the oven at 1100C
for about 30 minutes and allow it to cool in a dessicator.
2.) Weigh out 0.7 – 0.8 gram of KHP using the analytical balance.
3.) Dissolve the KHP in about 50 mL of cooled boiled water.
4.) Add 3 drops of phenolphthalein indicator.
5.) Titrate with the prepared NaOH until permanent faint pink color of solution is observed.
6.) Calculate the molarity of NaOH solution of up to 4 decimal places.
7.) Perform at least four trials; calculate the average molarity of NaOH and its standard deviation.
𝑔𝑟𝑎𝑚 𝐾𝐻𝑃 𝑥 1000
𝑁𝑁𝑎𝑂𝐻 = 𝑔
204.2212 𝑥 𝑉𝑁𝑎𝑂𝐻 (𝑖𝑛 𝑚𝐿)
𝑚𝑜𝑙
Determination of %KHP in an Impure Solid Sample
1.) Pass a clean amber bottle (labeled with your name) to your instructor.
2.) As soon as you receive the sample, your transfer it to a clean mortar and grind the mixture carefully.
Do not spill the sample.
3.) After grinding the sample, return it to the amber bottle.
4.) Heat the unknown sample in the oven at 110 0C for about 30 minutes.
5.) Allow it to cool in the dessicator.
6.) Weigh out 0.7 – 0.8 gram of the sample using the analytical balance.
7.) Dissolve the unknown sample in about 50 mL of cooled boiled water
8.) Add 3 drops of phenolphthalein indicator.
9.) Titrate with the prepared NaOH until permanent faint pink color of solution is observed.
10.) Perform at least 4 trials.
11.) Calculate the % KHP in the sample, the standard deviation, the % RSD of the experiment and the
critical limits at 95% confidence level.
DATA
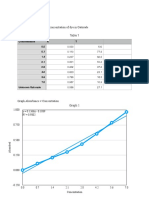
Table 1. Standardization of the Sodium Hydroxide solution
Volume
Standardizati mass KHP NaOH Molarity
Group on of NaOH (g) (mL) NaOH Average Std. Dev. % RSD
1 1 0.7464 33.30 0.1097 0.1083 0.0016 1.46
2 0.7461 34.10 0.1071
3 0.7480 33.60 0.1090
4 0.7449 34.20 0.1066
5 0.7485 33.20 0.1104
6 0.7501 34.30 0.1071
2 1 0.7806 35.30 0.1083 0.1083 0.0009 0.83
2 0.7303 33.00 0.1083
3 0.7338 33.20 0.1082
4 0.7162 32.40 0.1082
5 0.7268 33.30 0.1069
6 0.7483 33.40 0.1097
3 1 0.7058 32.50 0.1063 0.1077 0.0008 0.74
2 0.7646 34.60 0.1082
3 0.7498 34.20 0.1073
4 0.7121 32.10 0.1086
5 0.7319 33.20 0.1079
6 0.7572 34.40 0.1078
4 1 0.7232 33.50 0.1057 0.1039 0.0016 1.54
2 0.7194 33.50 0.1051
3 0.7363 34.90 0.1033
4 0.7336 35.50 0.1012
5 0.7778 36.70 0.1038
6 0.7035 33.00 0.1044
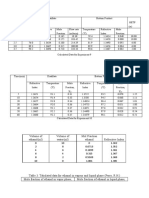
Table 2. Percent KHP in the sample
Volume
mass NaOH
Analysis 1 sample (g) (mL) % KHP Average Std. Dev. % RSD % Error
1 0.7031 22.20 69.85 69.64 0.23 0.34 0.00
2 0.7046 22.10 69.39
3 0.7049 22.20 69.67
True value 69.64
NaCl (g) 1.84
KHP (g) 4.22
1 0.7290 5.80 17.59 17.57 0.02 0.11 -68.64
2 0.7680 6.10 17.56
3 0.7305 5.80 17.56
True value 56.02
NaCl (g) 2.63
KHP (g) 3.35
1 0.7202 14.50 44.29 44.43 0.13 0.28 -0.20
2 0.7509 15.20 44.53
3 0.7222 14.60 44.47
True value 44.52
NaCl (g) 3.34
KHP (g) 2.68
1 0.7176 14.50 42.88 43.10 0.90 2.08 -3.18
2 0.7220 15.00 44.09 mb
3 0.7018 14.00 42.33
True value 44.52
NaCl (g) 3.34
KHP (g) 2.68
Table 3. Molar Mass of Unknown Monoprotic, Diprotic, and Triprotic Acids
Volume
mass NaOH Molar
Analysis 2 sample (g) (mL) Mass Average Std. Dev. % RSD % Error
1 0.7224 32.40 205.82 204.49 2.19 1.07 0.13
2 0.7219 32.40 205.68
3 0.7154 32.70 201.96
True value 204.22
1 0.7442 99.00 138.82 138.73 0.33 0.24 53.63
2 0.7527 100.00 139.00
3 0.7492 100.00 138.36
True value 90.30
1 0.7294 102.00 199.19 197.43 1.82 0.92 2.76
2 0.7425 104.70 197.54
3 0.7582 108.00 195.55
True value 192.12
1 0.7235 100.20 208.47 208.17 2.57 1.24 8.36
2 0.7145 100.40 205.47
3 0.7345 100.70 210.59
True value 192.12
ANALYSIS
CONCLUSION
To standardize the NaOH solution, it should be titrated with a weighed sample of KHP.
Standardized NaOH can be used for precise titrations of acid. The mass percent of KHP in an unknown
mixture is found by titrating a weighed sample of the mixture with the standardized NaOH solution. KHP
and NaOH has a 1:1 ratio. In determining the molar mass of an unknown acid, the moles of NaOH should
be determined with the volume and molarity of the standardized NaOH then determine the molar mass by
dividing the mass of the sample to the number of moles. For a monoprotic acid, it is only able to donate 1
proton per molecule which means it has a 1:1 ratio with the NaOH while diprotic acid has a 1:2 acid to base
ratio and triprotic acid has a 1:3 acid to base ratio.
You might also like
- Lab Report BoiDocument7 pagesLab Report BoiNORHIDAYATI BINTI MD GHAZALI MoeNo ratings yet
- CHM 3202 Lab#2Document13 pagesCHM 3202 Lab#2Raja GokhulNo ratings yet
- Government Publications: Key PapersFrom EverandGovernment Publications: Key PapersBernard M. FryNo ratings yet
- Abstract:: Na So +bacl2 Baso + 2naclDocument3 pagesAbstract:: Na So +bacl2 Baso + 2naclZahra Al-BasriNo ratings yet
- No Naoh (ML) K (MS/CM) Rata - Rata Data 1 Data 2Document8 pagesNo Naoh (ML) K (MS/CM) Rata - Rata Data 1 Data 2Teknik Kimia B 2016No ratings yet
- Results and Data CMT 463 Exp 3Document7 pagesResults and Data CMT 463 Exp 3IzzyanIsaNo ratings yet
- Antiscalant DosingDocument2 pagesAntiscalant DosingunconformistNo ratings yet
- Binary System Thermo DyncamixDocument19 pagesBinary System Thermo DyncamixJohn Fritz FestejoNo ratings yet
- Analisis Polinomial OrtogonalDocument48 pagesAnalisis Polinomial OrtogonalKhairul SamukiNo ratings yet
- Grupo de Asistencia Técnica S.R.LDocument2 pagesGrupo de Asistencia Técnica S.R.LLeandro BecerraNo ratings yet
- Granola Bar - Sheet1-2Document2 pagesGranola Bar - Sheet1-2api-447535032No ratings yet
- Spreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipDocument7 pagesSpreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipMohamed TallyNo ratings yet
- PreviewDocument17 pagesPreviewAdi KurdiNo ratings yet
- Tugas Kimia AnalitikDocument6 pagesTugas Kimia AnalitikIbal LaodeNo ratings yet
- Investigating The Effect of Change in Concentration On The Rate of ReactionDocument8 pagesInvestigating The Effect of Change in Concentration On The Rate of ReactionRitiNo ratings yet
- Chem 23 Lec Prob Set 2Document2 pagesChem 23 Lec Prob Set 2GenesisDaquinanNo ratings yet
- Contoh IKAAADocument57 pagesContoh IKAAABintang LestariNo ratings yet
- IA Sample 4 Students To Upload On MBDocument13 pagesIA Sample 4 Students To Upload On MBSiddhesh SoodNo ratings yet
- Reference Simulation: Project Information: Case-Specific: System DetailsDocument17 pagesReference Simulation: Project Information: Case-Specific: System Detailssoe0303No ratings yet
- Annexure B - Sucrose Conversion TableDocument21 pagesAnnexure B - Sucrose Conversion TableLim Chen Gin100% (1)
- Esterification of EthanolDocument15 pagesEsterification of EthanolSadia HasanNo ratings yet
- Lampiran B Contoh Perhitungan: B.1 Membuat Larutan 200 ML Naoh 1 NDocument5 pagesLampiran B Contoh Perhitungan: B.1 Membuat Larutan 200 ML Naoh 1 NImmanuel HutagaolNo ratings yet
- 4 RO Pilot ScaleDocument2 pages4 RO Pilot ScaleunconformistNo ratings yet
- Chem 112.1 - Exer 9 Table and AnswersDocument7 pagesChem 112.1 - Exer 9 Table and AnswersGerry Mark GubantesNo ratings yet
- Viskositas Vs PH: PH Buffer PH Ukur (CP) (G.ML)Document3 pagesViskositas Vs PH: PH Buffer PH Ukur (CP) (G.ML)Vira ValasaraNo ratings yet
- Lab Sheet 7 Lab BeatingDocument39 pagesLab Sheet 7 Lab BeatingWan Aziz Wan Othman0% (1)
- Excel HysysDocument11 pagesExcel HysysAndrie Kurniawan IndraNo ratings yet
- Anachem-Lab-Vol-Method - SILVERIO-BSMT2GDocument3 pagesAnachem-Lab-Vol-Method - SILVERIO-BSMT2GMiggy PascualNo ratings yet
- BCA Analysis - Lab Report - 40406647Document6 pagesBCA Analysis - Lab Report - 40406647Janavi MotwaniNo ratings yet
- Chem Food Lab Dye Paper WDocument2 pagesChem Food Lab Dye Paper Wapi-345228043No ratings yet
- Potentiometric PH Measurement Lab ReportDocument6 pagesPotentiometric PH Measurement Lab Reportrhima shineyNo ratings yet
- 21-04 - Aurora Martha Herdiani - Data Sheet MO 5Document12 pages21-04 - Aurora Martha Herdiani - Data Sheet MO 5FAUZAN HANIF AL FIKRI FAUZAN HANIF AL FIKRINo ratings yet
- No. Parameter Satuan Baku Mutu (PP No.22 Th.2021) Nilai Maksimal Nilai Minimal FisikaDocument2 pagesNo. Parameter Satuan Baku Mutu (PP No.22 Th.2021) Nilai Maksimal Nilai Minimal FisikaREZKY WAHYUDINo ratings yet
- Phosphoric Acid Titration CurveDocument4 pagesPhosphoric Acid Titration Curvedavid.anthony010700No ratings yet
- Thermodynamic Functions and Solubility Product of Barium NitrateDocument11 pagesThermodynamic Functions and Solubility Product of Barium NitrateNabilah HarisNo ratings yet
- Result and CalculationDocument13 pagesResult and CalculationgerrykhangNo ratings yet
- Water Lab ReportDocument8 pagesWater Lab ReportMonica GellerNo ratings yet
- Chemical Reaction Engineering Lab BKF3741 2019/2020 SEMESTER 2Document15 pagesChemical Reaction Engineering Lab BKF3741 2019/2020 SEMESTER 2Syazana SabriNo ratings yet
- Kurva Titrasi Basa Kuat Oleh Asam Kuat: Volume HNO3 (ML)Document4 pagesKurva Titrasi Basa Kuat Oleh Asam Kuat: Volume HNO3 (ML)MaulinaNo ratings yet
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 2: Enzyme Assays and Factors Affecting Enzyme Activity Full Lab ReportDocument10 pagesCPB 30103 Biochemical Engineering UniKL MICET Experiment 2: Enzyme Assays and Factors Affecting Enzyme Activity Full Lab ReportSiti Hajar MohamedNo ratings yet
- Calculadora Indice de LangelierDocument6 pagesCalculadora Indice de LangelierFábio SenaNo ratings yet
- 0.1 2020support 1Document16 pages0.1 2020support 1Aparna PrasadNo ratings yet
- PH Calculo y Estimacion de Incertidumbre - REMARCODocument8 pagesPH Calculo y Estimacion de Incertidumbre - REMARCOFran GuidoNo ratings yet
- Naocl Examples PDFDocument5 pagesNaocl Examples PDFBasu RayalaNo ratings yet
- Registro TelarañaDocument9 pagesRegistro TelarañaMartin de Jesus Olamendi VergaraNo ratings yet
- Example Calculations of Chlorine DosageDocument5 pagesExample Calculations of Chlorine DosageMuammar QadafiNo ratings yet
- 978 1 941926 24 6 - Chapter03Document14 pages978 1 941926 24 6 - Chapter03Federico GaleanoNo ratings yet
- Analytical 3Document6 pagesAnalytical 3Seyram DavidNo ratings yet
- SBL1023 Techniques in Biological and Biochemistry LaboratoryDocument9 pagesSBL1023 Techniques in Biological and Biochemistry Laboratoryapi-383715002No ratings yet
- R. 25,0C 0,0y 75,0R 28-09-18Document2 pagesR. 25,0C 0,0y 75,0R 28-09-18unconformistNo ratings yet
- Perubahan Bobot: Perlakuan Ulangan Awal Akhir Selisih SD (%)Document9 pagesPerubahan Bobot: Perlakuan Ulangan Awal Akhir Selisih SD (%)efendiNo ratings yet
- Group 6 Feed Formulation For Chicken LayerDocument5 pagesGroup 6 Feed Formulation For Chicken Layercheryl rose vidalNo ratings yet
- Solubility Curve Practice Problems Worksheet 1Document3 pagesSolubility Curve Practice Problems Worksheet 1judith cueNo ratings yet
- IRMAWATI 1814241012 NTP (Tugas INTU Nyusun Ransum)Document7 pagesIRMAWATI 1814241012 NTP (Tugas INTU Nyusun Ransum)Ratu Haulah Kholillah YusufNo ratings yet
- Practical BiochemistryDocument5 pagesPractical BiochemistrylinhNo ratings yet
- Ion Selective ElectrodesDocument11 pagesIon Selective ElectrodesOm PhileNo ratings yet
- 100 Well 1 Well 2 Well 1 Well 2Document4 pages100 Well 1 Well 2 Well 1 Well 2hari6622No ratings yet
- Solubility Curve Practice Problems Worksheet 1Document2 pagesSolubility Curve Practice Problems Worksheet 1Unexpected TheoryNo ratings yet
- Enabling Task 1-Background of The Study: International Reference/Source National Reference/Source Local Reference/SourceDocument4 pagesEnabling Task 1-Background of The Study: International Reference/Source National Reference/Source Local Reference/SourceSamantha Jesi Shania EstradaNo ratings yet
- Bonifacio Before The Revolution NotesDocument5 pagesBonifacio Before The Revolution NotesSamantha Jesi Shania EstradaNo ratings yet
- Bonifacio Before The Revolution NotesDocument5 pagesBonifacio Before The Revolution NotesSamantha Jesi Shania EstradaNo ratings yet
- Bonifacio Before The Revolution NotesDocument5 pagesBonifacio Before The Revolution NotesSamantha Jesi Shania EstradaNo ratings yet
- Enabling Task 2 - Making HypothesisDocument2 pagesEnabling Task 2 - Making HypothesisSamantha Jesi Shania EstradaNo ratings yet
- RPH Lecture 1: Introduction To Readings in Philippine HistoryDocument2 pagesRPH Lecture 1: Introduction To Readings in Philippine HistorySamantha Jesi Shania EstradaNo ratings yet
- FA - JESSIE MARTIN ESTRADA - Practical Work 1Document4 pagesFA - JESSIE MARTIN ESTRADA - Practical Work 1Samantha Jesi Shania EstradaNo ratings yet
- Enabling Task 2 - Making HypothesisDocument2 pagesEnabling Task 2 - Making HypothesisSamantha Jesi Shania EstradaNo ratings yet
- Text 1 Title: I Have A Dream By: Martin Luther King Jr. Text 2 by Edgar Allan Poe Text 3 by Columbia PicturesDocument3 pagesText 1 Title: I Have A Dream By: Martin Luther King Jr. Text 2 by Edgar Allan Poe Text 3 by Columbia PicturesSamantha Jesi Shania EstradaNo ratings yet
- Enabling Task 1-Background of The Study: International Reference/Source National Reference/Source Local Reference/SourceDocument4 pagesEnabling Task 1-Background of The Study: International Reference/Source National Reference/Source Local Reference/SourceSamantha Jesi Shania EstradaNo ratings yet
- Scaffold 1A - Background of The StudyDocument4 pagesScaffold 1A - Background of The StudySamantha Jesi Shania EstradaNo ratings yet
- Solution PrepDocument15 pagesSolution PrepKhaled Saif AldinNo ratings yet
- Scaffold 1A - Background of The StudyDocument4 pagesScaffold 1A - Background of The StudySamantha Jesi Shania EstradaNo ratings yet
- Text 1 Title: I Have A Dream By: Martin Luther King Jr. Text 2 by Edgar Allan Poe Text 3 by Columbia PicturesDocument3 pagesText 1 Title: I Have A Dream By: Martin Luther King Jr. Text 2 by Edgar Allan Poe Text 3 by Columbia PicturesSamantha Jesi Shania EstradaNo ratings yet
- Ver1 PDFDocument37 pagesVer1 PDFSamantha Jesi Shania EstradaNo ratings yet
- Kami Export - Scaffold 1bDocument5 pagesKami Export - Scaffold 1bSamantha Jesi Shania EstradaNo ratings yet
- Title: I Have A Dream Title: The Lottery Ticket Title 3: The PatriotDocument2 pagesTitle: I Have A Dream Title: The Lottery Ticket Title 3: The PatriotSamantha Jesi Shania EstradaNo ratings yet
- Neutralization TitrationDocument2 pagesNeutralization TitrationSamantha Jesi Shania EstradaNo ratings yet
- Pink and Cream Illustration Social Science Class Education PresentationDocument4 pagesPink and Cream Illustration Social Science Class Education PresentationSamantha Jesi Shania EstradaNo ratings yet
- Kami Export - 3Q - GGTemplateDocument1 pageKami Export - 3Q - GGTemplateSamantha Jesi Shania EstradaNo ratings yet
- Ver1 PDFDocument37 pagesVer1 PDFSamantha Jesi Shania EstradaNo ratings yet
- Lesson 13. SEEDDocument3 pagesLesson 13. SEEDSamantha Jesi Shania EstradaNo ratings yet
- HakeemsAngels OSMOSISDocument5 pagesHakeemsAngels OSMOSISSamantha Jesi Shania EstradaNo ratings yet
- Kami Export - Scaffold #1a Organizing Your RRLDocument3 pagesKami Export - Scaffold #1a Organizing Your RRLSamantha Jesi Shania EstradaNo ratings yet
- Circulatory System of A FrogDocument23 pagesCirculatory System of A FrogSamantha Jesi Shania Estrada100% (1)
- Analytical Chemistry: Precipitate - Turn The Soluble Substance Into An Insoluble SubstanceDocument7 pagesAnalytical Chemistry: Precipitate - Turn The Soluble Substance Into An Insoluble SubstanceSamantha Jesi Shania EstradaNo ratings yet
- Factors That Affect The Rate of PhotosynthesisDocument7 pagesFactors That Affect The Rate of PhotosynthesisSamantha Jesi Shania Estrada100% (1)
- Online Treasure HuntDocument2 pagesOnline Treasure HuntSamantha Jesi Shania EstradaNo ratings yet
- Topic 5 Adolescent BrainDocument1 pageTopic 5 Adolescent BrainSamantha Jesi Shania EstradaNo ratings yet
- A675/A675MDocument5 pagesA675/A675Mpavan_joshi_5100% (1)
- Cations and AnionsDocument22 pagesCations and AnionsDoe BlackNo ratings yet
- Index: Combustion Technology: Essential of Flames and Burners, First Edition SDocument3 pagesIndex: Combustion Technology: Essential of Flames and Burners, First Edition SirNo ratings yet
- 2018 CTI Paper - No Charge - A Novel Non-IonicDocument18 pages2018 CTI Paper - No Charge - A Novel Non-IonicMike StandishNo ratings yet
- Gregory D. Botsaris (Auth.), J. W. Mullin (Eds.) - Industrial Crystallization-Springer US (1976) PDFDocument456 pagesGregory D. Botsaris (Auth.), J. W. Mullin (Eds.) - Industrial Crystallization-Springer US (1976) PDFAlan ConnorNo ratings yet
- Water Treatment DissertationDocument8 pagesWater Treatment DissertationNeedHelpWithPaperErie100% (1)
- ExamView - Sch4u Organic TestDocument6 pagesExamView - Sch4u Organic TestMahir AhmedNo ratings yet
- Smith and Parsons, 1973Document3 pagesSmith and Parsons, 1973aaNo ratings yet
- Wall - Panel - KS1150 TF - NF - DatasheetDocument4 pagesWall - Panel - KS1150 TF - NF - DatasheetMetlachisNo ratings yet
- Activity 2 - Biochemical Processes (Revised 6.8.20)Document6 pagesActivity 2 - Biochemical Processes (Revised 6.8.20)Sherma Sheikh karimNo ratings yet
- Vicks Vaporizer v100Document2 pagesVicks Vaporizer v100eddieyetNo ratings yet
- Pro Safety MCQSDocument87 pagesPro Safety MCQSAtif ZahidNo ratings yet
- 32M 06004 01.metric PDFDocument66 pages32M 06004 01.metric PDFErdian FakirNo ratings yet
- 42) API 653 DAY 5 BOOK (1 To 58)Document58 pages42) API 653 DAY 5 BOOK (1 To 58)SHAHIDALI100% (2)
- Tempering MartensiteDocument21 pagesTempering Martensitejardel de matosNo ratings yet
- Material Sub Group Item CodeDocument2,818 pagesMaterial Sub Group Item Codegouri gouriNo ratings yet
- Specification of Rural RoadsDocument70 pagesSpecification of Rural RoadsMadhavpokaleNo ratings yet
- UntitledDocument463 pagesUntitledtheopascuNo ratings yet
- CHEM2212 - ESTANOCO - SAMUEL.M. - Activity 1 LABORATORY EQUIPMENTDocument6 pagesCHEM2212 - ESTANOCO - SAMUEL.M. - Activity 1 LABORATORY EQUIPMENTSam EstanocoNo ratings yet
- "Oil and Gas Processing Plant Design and Operation Training Course" " " 2002Document10 pages"Oil and Gas Processing Plant Design and Operation Training Course" " " 2002AjaykumarNo ratings yet
- Quantitative Estimation of Vitamin C (Ascorbic Acid) by IodimetryDocument9 pagesQuantitative Estimation of Vitamin C (Ascorbic Acid) by IodimetryDeepak PradhanNo ratings yet
- Science of The Total EnvironmentDocument14 pagesScience of The Total EnvironmentKArenNo ratings yet
- The Langmuir IsothermDocument12 pagesThe Langmuir IsothermAlejandro MartinNo ratings yet
- InorganichwDocument7 pagesInorganichwArvit CtkhuNo ratings yet
- Calculations Using Tanabe-Sugano DiagramsDocument7 pagesCalculations Using Tanabe-Sugano DiagramsFANDOMNo ratings yet
- Effect of Ggbs & Copper Slag With The Partial Replacement of Cement & Fine Aggregate in PQC MixDocument11 pagesEffect of Ggbs & Copper Slag With The Partial Replacement of Cement & Fine Aggregate in PQC Mixkunal pawarNo ratings yet
- 2013 Book BiotechnologyForMedicinalPlantDocument465 pages2013 Book BiotechnologyForMedicinalPlantKenya100% (1)
- AB Emultech Private LimitedDocument18 pagesAB Emultech Private LimitedamistalokNo ratings yet
- Mil C 7438Document28 pagesMil C 7438sohail goharNo ratings yet
- Tds 108277427 ProMix PCE 300Document3 pagesTds 108277427 ProMix PCE 300Chetal BholeNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- Pocket Guide to Flanges, Fittings, and Piping DataFrom EverandPocket Guide to Flanges, Fittings, and Piping DataRating: 3.5 out of 5 stars3.5/5 (22)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- Phase Equilibria in Chemical EngineeringFrom EverandPhase Equilibria in Chemical EngineeringRating: 4 out of 5 stars4/5 (11)