Professional Documents
Culture Documents
1 PDF
1 PDF
Uploaded by
Rohit Shrestha0 ratings0% found this document useful (0 votes)
13 views2 pagesOriginal Title
1 (54).pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views2 pages1 PDF
1 PDF
Uploaded by
Rohit ShresthaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Air Conditioning Systems 55
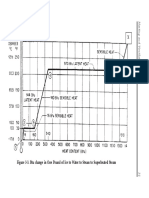
water, which incidentally is also a refrigerant (refrigerant-718), is
that the boiling point of our refrigerant is minus forty degrees be-
low zero (-40), while the boiling point of water is 212 degrees
above zero. Both these boiling points occur at sea level. It is im-
portant to understand that the boiling point of a liquid will
change in the same direction as the pressure to which the liquid
is subjected. For example, water at sea level, 14.7 pounds per
square inch, boils at 212°F, while water subjected to 25 pounds per
square inch of pressure boils at approximately 240°F. Since our
closed refrigeration system is under pressure, in other words
greater than atmospheric, we have elevated the boiling point of
the refrigerant to approximately 40°F above zero. As the refriger-
ant passes through the evaporator tubes the boiling process con-
tinues. As long as the refrigerant is changing state from a liquid
to a vapor the temperature remains at 40°F. However, once all the
liquid has been changed to a vapor, and this occurs near the end
of the evaporator, the vapor can now absorb additional heat. This
process is called superheating the vapor, or simply, superheat.
Our system will pick up about 10 degrees of superheat and
the refrigerant, which is now a low-pressure, low-temperature
vapor, will flow through the suction line and enter the compres-
sion stage at 50°F. The compression stage consists of an electri-
cally driven mechanical compressor. The compressor has two
main functions within the refrigeration cycle. One function is to
pump the refrigerant vapor from the evaporator so that the de-
sired temperature and pressure can be maintained in the evapo-
rator. The second function is to increase the pressure of the
refrigerant vapor through the process of compression, and simul-
taneously increase the temperature of the vapor. This change in
pressures also causes the refrigerant to flow through the system.
Let’s say that our compressor increases the pressure of the vapor
so that the corresponding temperature of the vapor will be 120°F.
This is the condensing temperature, that is, the temperature in the
condenser. This high-pressure, high-temperature vapor leaves the
compressor and enters the condensation stage. In our example,
the actual temperature of the refrigerant in the hot gas or dis-
56 HVAC Fundamentals
charge line is 170°F. The temperature of the refrigerant will cool
down from 170°F to 120°F as it goes through the hot gas line and
in the condenser. This loss of heat, in this case 50°F of sensible
heat, is called “desuperheating.”
The condensation stage in our refrigeration system consists
of an air-cooled condenser coil and a fan. Some systems however,
use a pump and a water-cooled condenser. Our air-cooled con-
denser has a fan or blower, sometimes called the outdoor fan,
which draws outside air across the condenser coil. The tempera-
ture of the refrigerant vapor flowing through the condenser tubes
is 120°F. At the same time, the 90°F outside air is passing over the
condenser tubes. As before, heat travels from a higher tempera-
ture to a lower temperature. Since the air passing over the con-
denser coil is cooler than the refrigerant in the tubes, heat will be
picked up by the outside air. In other words, the refrigerant is
cooled and the air is heated. The condenser is said to be discharg-
ing or rejecting its heat into the atmosphere.
Let’s back up for a minute. Where did we get this heat that
is in the condenser? Well, about 75% of it is the unwanted heat
from the conditioned space. The other 25% is heat from the com-
pression stage. So now we have taken the unwanted heat from
one place, the conditioned space, and discharged it to another
place, the outside.
In order for the refrigerant to be able to pick up more heat
from the supply air it must once again become a low-temperature
liquid. The cooling of the vapor in the condenser causes the re-
frigerant to change state from a vapor to a liquid. This process is
called condensation. As the refrigerant vapor passes through the
tubes the condensation process continues. As long as the refriger-
ant is changing state from a vapor to a liquid the temperature
remains at 120°F.
However, once the entire vapor has been changed to liquid,
the liquid can reject additional heat. As the refrigerant, which is
now a high-pressure, high temperature liquid (120°F @ 260 psig)
flows through the liquid line to the pressure reducing device it
continues to give up heat. This is called “subcooling.” The liquid
You might also like
- Air Conditioning Systems 57Document2 pagesAir Conditioning Systems 57rohitNo ratings yet
- Refrigeration CycleDocument5 pagesRefrigeration CycleMa Zaira ObilloNo ratings yet
- Water Chillers 85Document2 pagesWater Chillers 85Rohit ShresthaNo ratings yet
- Super Heat and Sub CoolingDocument2 pagesSuper Heat and Sub CoolingMuhammad BilalNo ratings yet
- Aircon NotesDocument5 pagesAircon Notesprado01No ratings yet
- Refrigeration CycleDocument5 pagesRefrigeration CycleLiezel Quijada LicupNo ratings yet
- Group 5 Melab3Document14 pagesGroup 5 Melab3Gigi SalesNo ratings yet
- Hvac Lab Report: Name: Ali Raza Roll No: 18-MCE-18 Submitted To: Sir Murawat Abbas Semester 6Document11 pagesHvac Lab Report: Name: Ali Raza Roll No: 18-MCE-18 Submitted To: Sir Murawat Abbas Semester 6AliNo ratings yet
- Refrigeration Cycle DiagramDocument7 pagesRefrigeration Cycle DiagramsrybsantosNo ratings yet
- Refrigeration Systems: Home License Page Engineer Exam Questions Deck Exam QuestionsDocument20 pagesRefrigeration Systems: Home License Page Engineer Exam Questions Deck Exam QuestionsDennis ErminoNo ratings yet
- Vapor Compression Refrigeration CycleDocument21 pagesVapor Compression Refrigeration CycleUSHA PAWARNo ratings yet
- Evaporator: Fan SpeedsDocument2 pagesEvaporator: Fan SpeedsPradeep SukumaranNo ratings yet
- Physics 2Document5 pagesPhysics 2SOLAIMANNo ratings yet
- Refrigeration Cycle, HVAC System Basics and Refrigerant Charging PDFDocument13 pagesRefrigeration Cycle, HVAC System Basics and Refrigerant Charging PDFMurillo MendesNo ratings yet
- Reerigeation System Basics, Week 2 Part 1Document17 pagesReerigeation System Basics, Week 2 Part 1Musfirah AdeelNo ratings yet
- Condenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToDocument37 pagesCondenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToAliceNo ratings yet
- The Importance of A Clean CondenserDocument5 pagesThe Importance of A Clean CondenserD HarNo ratings yet
- Parts of A Refrigeration SystemDocument8 pagesParts of A Refrigeration SystemRay RavelNo ratings yet
- Refrigeration 1Document13 pagesRefrigeration 1Vishwanathan RishanthNo ratings yet
- REFRIGERATIONDocument24 pagesREFRIGERATIONDark ShadyNo ratings yet
- RefrigerationDocument65 pagesRefrigerationyassinNo ratings yet
- 3.4 Refrigeration Systems (1) - 1Document2 pages3.4 Refrigeration Systems (1) - 1Beena RawatNo ratings yet
- Study of RefrigiratorDocument14 pagesStudy of RefrigiratorRobo RajaNo ratings yet
- Heat Pump TrainerDocument6 pagesHeat Pump TrainerAfzaal FiazNo ratings yet
- Refregeration UnitDocument24 pagesRefregeration UnitEZWANNo ratings yet
- Mechanical-Compression Refrigeration SystemsDocument4 pagesMechanical-Compression Refrigeration Systemsaruna MoonNo ratings yet
- Performance and Efficiency Test of Refrigeration PlantDocument26 pagesPerformance and Efficiency Test of Refrigeration PlantGigi Sales100% (2)
- Refrigeration With Hot and Cooled BoxDocument69 pagesRefrigeration With Hot and Cooled Boxkris_soneNo ratings yet
- HVAC and Refrigeration SystemDocument25 pagesHVAC and Refrigeration SystemZoya ShaikhNo ratings yet
- Basic Refrigeration CycleDocument14 pagesBasic Refrigeration Cyclehussein alnasryNo ratings yet
- Performance and Efficiency Test of A Refrigeration PlantDocument17 pagesPerformance and Efficiency Test of A Refrigeration Plantjun75% (4)
- WATER COOLER 9235074-F935-49bc-90cc-08ed4ab8014dDocument3 pagesWATER COOLER 9235074-F935-49bc-90cc-08ed4ab8014dAmbika prasadNo ratings yet
- Vapour Compression CycleDocument16 pagesVapour Compression CycleHrishikesh ShindeNo ratings yet
- Refrigeration CycleDocument29 pagesRefrigeration CycleShyam WanaskarNo ratings yet
- FridgeDocument6 pagesFridgeshashlearnNo ratings yet
- Refrigeration Unit Lab ReportDocument28 pagesRefrigeration Unit Lab ReportShinigdho Synthia79% (14)
- Functioning of ChillersDocument22 pagesFunctioning of ChillersAnonymous b9fcR5No ratings yet
- Absorption Refrigeration SystemsDocument4 pagesAbsorption Refrigeration SystemspraneshNo ratings yet
- Air Conditioning Theory (Automotive)Document21 pagesAir Conditioning Theory (Automotive)ingenierosunidosNo ratings yet
- Ref BasicsDocument15 pagesRef BasicsManasvini KarthihaNo ratings yet
- ChatGPT Heat Vs Temp.Document7 pagesChatGPT Heat Vs Temp.Ali Husnain ShadNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- Refrigeration and Air Conditioning (7.2.22)Document148 pagesRefrigeration and Air Conditioning (7.2.22)Edwin Abregú Leandro100% (1)
- Vapor Compression Refrigeration SystemDocument6 pagesVapor Compression Refrigeration SystemGerson Paul BangoyNo ratings yet
- Part 3 Phase Change of The Refrigerant in The CycleDocument7 pagesPart 3 Phase Change of The Refrigerant in The CycleKenneth JameroNo ratings yet
- Refrig Terms GoodDocument6 pagesRefrig Terms GoodAbhilashNo ratings yet
- Performance and Efficiency Test of A Refrigeration PlantDocument17 pagesPerformance and Efficiency Test of A Refrigeration PlantAllen Espeleta0% (1)
- Group 2 Basic Refrigerationsystem and ComponentsDocument25 pagesGroup 2 Basic Refrigerationsystem and ComponentsVipin VetriNo ratings yet
- What Is The Basic Principle of Refrigeration and What Do You Understand by Refrigeration Cycle?Document3 pagesWhat Is The Basic Principle of Refrigeration and What Do You Understand by Refrigeration Cycle?a4104165No ratings yet
- Rac Solution Set ADocument7 pagesRac Solution Set AMuhammad AkhtarNo ratings yet
- Fundamentals 1 CycleDocument1 pageFundamentals 1 Cyclepali_peNo ratings yet
- Facility Maintenance 1Document163 pagesFacility Maintenance 1Ashok AmnarNo ratings yet
- 18-Tugas 1-Prak. Perawatan Mesin Konversi Energi 1Document5 pages18-Tugas 1-Prak. Perawatan Mesin Konversi Energi 1Oh WiwinNo ratings yet
- 4 Performance and Efficiency Test of A Refrigeration PlantDocument52 pages4 Performance and Efficiency Test of A Refrigeration PlantIvy Joy UbinaNo ratings yet
- Building ServicesDocument154 pagesBuilding ServicesjeevaNo ratings yet
- Solar Automobile FridgeDocument4 pagesSolar Automobile FridgeRahul SinghNo ratings yet
- Major Elements of Refrigeration System and Their FunctionsDocument8 pagesMajor Elements of Refrigeration System and Their FunctionsXahid HasanNo ratings yet
- Types of ChillerDocument13 pagesTypes of Chillerzakaria masud sonyNo ratings yet
- Part 3 Phase Change of The Refrigerant in The CycleDocument7 pagesPart 3 Phase Change of The Refrigerant in The CycleKenneth JameroNo ratings yet
- 1 PDFDocument2 pages1 PDFrohitNo ratings yet
- Heating and Ventilating Systems 25Document2 pagesHeating and Ventilating Systems 25rohitNo ratings yet
- Figure 3-3. Btu Change in One Pound of Ice To Water To Steam To Superheated SteamDocument2 pagesFigure 3-3. Btu Change in One Pound of Ice To Water To Steam To Superheated SteamrohitNo ratings yet
- Heating and Ventilating Systems 33Document2 pagesHeating and Ventilating Systems 33rohitNo ratings yet
- Heating and Ventilating Systems 35Document2 pagesHeating and Ventilating Systems 35rohitNo ratings yet
- Figure 4-4. Air-to-Water AC SystemDocument2 pagesFigure 4-4. Air-to-Water AC SystemrohitNo ratings yet
- Heating and Ventilating Systems 29: Figure 3-2. Steam BoilerDocument2 pagesHeating and Ventilating Systems 29: Figure 3-2. Steam BoilerrohitNo ratings yet
- Heating and Ventilating Systems 37: Figure 3-6. Combustion Chamber and Fire Tubes. Two-Pass BoilerDocument2 pagesHeating and Ventilating Systems 37: Figure 3-6. Combustion Chamber and Fire Tubes. Two-Pass BoilerrohitNo ratings yet
- Heating and Ventilating Systems 27Document2 pagesHeating and Ventilating Systems 27rohitNo ratings yet
- Air Conditioning Systems 63: Evaporators (Heat Picked Up From The Conditioned Space)Document2 pagesAir Conditioning Systems 63: Evaporators (Heat Picked Up From The Conditioned Space)rohitNo ratings yet
- Btuh GPM ×: Heat Flow 23Document2 pagesBtuh GPM ×: Heat Flow 23rohitNo ratings yet
- Air Conditioning Systems 59: Figure 4-3. Water-to-Water AC SystemDocument2 pagesAir Conditioning Systems 59: Figure 4-3. Water-to-Water AC SystemrohitNo ratings yet
- Figure 4-.2 Air Conditioning System ExampleDocument2 pagesFigure 4-.2 Air Conditioning System ExamplerohitNo ratings yet
- Figure 4-1. Central HVAC System "Air Conditioning"Document2 pagesFigure 4-1. Central HVAC System "Air Conditioning"rohitNo ratings yet
- Air Conditioning Systems 47Document2 pagesAir Conditioning Systems 47rohitNo ratings yet
- Air Conditioning Systems 51Document2 pagesAir Conditioning Systems 51rohitNo ratings yet
- Figure 3-10. Central HVAC System "Ventilating"Document2 pagesFigure 3-10. Central HVAC System "Ventilating"rohitNo ratings yet
- Latent HeatDocument2 pagesLatent HeatrohitNo ratings yet
- Heating and Ventilating Systems 43: MAT (%OA ×Document2 pagesHeating and Ventilating Systems 43: MAT (%OA ×rohitNo ratings yet
- Heating and Ventilating Systems 41: Figure 3-8. Oil BurnerDocument2 pagesHeating and Ventilating Systems 41: Figure 3-8. Oil BurnerrohitNo ratings yet
- This Page Intentionally Left BlankDocument2 pagesThis Page Intentionally Left BlankrohitNo ratings yet
- HVAC Systems 1Document2 pagesHVAC Systems 1rohitNo ratings yet
- Heat Flow 19Document2 pagesHeat Flow 19rohitNo ratings yet
- Heat Flow 17: ConductionDocument2 pagesHeat Flow 17: ConductionrohitNo ratings yet
- This Page Intentionally Left BlankDocument2 pagesThis Page Intentionally Left BlankrohitNo ratings yet
- Heat Flow 15Document2 pagesHeat Flow 15rohitNo ratings yet
- HVAC Systems 13: VentilatingDocument2 pagesHVAC Systems 13: VentilatingrohitNo ratings yet
- Air Volume: HVAC Systems 11Document2 pagesAir Volume: HVAC Systems 11rohitNo ratings yet
- HVAC Systems 9Document2 pagesHVAC Systems 9rohitNo ratings yet
- Naca 4412Document19 pagesNaca 4412Ashfaq AhamedNo ratings yet
- Accumulator Capacity - Usable Volume Per BottleDocument2 pagesAccumulator Capacity - Usable Volume Per BottleEslam Atif AzkolNo ratings yet
- Project ReportDocument62 pagesProject ReportAll_regNo ratings yet
- HH 102 SemitrailerDocument6 pagesHH 102 SemitrailerleoNo ratings yet
- Indices MecânicosDocument9 pagesIndices MecânicosSildes Lapa Corsini JuniorNo ratings yet
- A Study On The Floating Bridge Type Horizontal Axis Tidal Current Turbine For Energy Independent Islands in KoreaDocument14 pagesA Study On The Floating Bridge Type Horizontal Axis Tidal Current Turbine For Energy Independent Islands in KoreaNisa AhmadNo ratings yet
- Hydraulic CalculationDocument7 pagesHydraulic CalculationRais Rijal100% (1)
- EMP Bentu Process Flow 2022Document1 pageEMP Bentu Process Flow 2022Maintenance BentuNo ratings yet
- PIP PN03SD0B02 Piping Material Specification 3SD0B02 Class 300, 316/316L Stainless Steel, Butt Weld, 0.000 C.A., Process (PTFE Packing/Gaskets)Document5 pagesPIP PN03SD0B02 Piping Material Specification 3SD0B02 Class 300, 316/316L Stainless Steel, Butt Weld, 0.000 C.A., Process (PTFE Packing/Gaskets)César SantanaNo ratings yet
- Assignment#1Document5 pagesAssignment#1Hennesey LouriceNo ratings yet
- Uv Series Data Sheet: "H" VersionDocument1 pageUv Series Data Sheet: "H" VersionCapacitacion TodocatNo ratings yet
- Double Ball ValveDocument5 pagesDouble Ball ValveValmac ServicesNo ratings yet
- Tension Variations of Hydro-Pneumatic Riser Tensioner and Implications For Dry-Tree Interface in SemisubmersibleDocument18 pagesTension Variations of Hydro-Pneumatic Riser Tensioner and Implications For Dry-Tree Interface in SemisubmersibleJuan Alejandro Cañas ColoradoNo ratings yet
- Transmission Control Valve: Operación de SistemasDocument11 pagesTransmission Control Valve: Operación de Sistemasgalvis1020100% (1)
- Tetra Cardboard Packer 70Document808 pagesTetra Cardboard Packer 70Omar AlneasNo ratings yet
- Inspection Punch ListDocument2 pagesInspection Punch Listjrod915No ratings yet
- Reverse Circulation Pump PDFDocument4 pagesReverse Circulation Pump PDFthawdarNo ratings yet
- Compressor - Problem SolvingDocument11 pagesCompressor - Problem SolvingLorenz Banada0% (1)
- Fundamentals of Gas Pipeline Metering StationsDocument5 pagesFundamentals of Gas Pipeline Metering StationsSarah DeanNo ratings yet
- Experiment Multi Pump Test RigDocument55 pagesExperiment Multi Pump Test RigSurendran Balakrishnan88% (16)
- Ivy Catalogue From ERIKCDocument33 pagesIvy Catalogue From ERIKCGiovaniBalzani100% (3)
- Convection Heat Transfer (Chapter 6)Document25 pagesConvection Heat Transfer (Chapter 6)Ahmad Aiman ZaharinNo ratings yet
- RESULT, DISCUSSION, Calculation, ConclusionDocument11 pagesRESULT, DISCUSSION, Calculation, ConclusionHafizszul Feyzul80% (5)
- 08 Viscous FlowDocument40 pages08 Viscous FlowJohn DoeNo ratings yet
- Basic Types of Fans Used For Ventilating Underground MinesDocument15 pagesBasic Types of Fans Used For Ventilating Underground MinesNag Raj RockssNo ratings yet
- Spirex Sarco Piston Acctuated ValveDocument12 pagesSpirex Sarco Piston Acctuated ValveAhmed MoharramNo ratings yet
- Regenerative Feed Heating System: RegenerationDocument2 pagesRegenerative Feed Heating System: RegenerationShounak DasNo ratings yet
- Turbine Auxilaries Q A PDFDocument22 pagesTurbine Auxilaries Q A PDFGanesh DasaraNo ratings yet
- REL-BFW-300 FM & UL Wafer Butterfly Valve: Material List Component MaterialDocument2 pagesREL-BFW-300 FM & UL Wafer Butterfly Valve: Material List Component MaterialSavy PhanethNo ratings yet
- Department of Mechanical Engineering: List of Experiments Hvac LabDocument3 pagesDepartment of Mechanical Engineering: List of Experiments Hvac LabHamid MasoodNo ratings yet