Professional Documents
Culture Documents
ws16 Diastereomers Professor Jennifer Poutsma PDF
Uploaded by
Sankar AdhikariOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ws16 Diastereomers Professor Jennifer Poutsma PDF
Uploaded by
Sankar AdhikariCopyright:
Available Formats
lOMoARcPSD|5381057
WS16, CHEM 211, Dr. Poutsma 1
Worksheet 16
Diastereomers
What is the relationship between non-superimposable isomers that are not mirror images?
Model 1: Diastereomers
Diastereomers are non-superimposable stereoisomers (i.e. have the same connectivity, but different
arrangement in space) and are NOT MIRROR IMAGES!!!
CH3 CH3 CH3 CH3
Br S Br Br S Br
S I I I I
R R
A Cl B Cl C Cl D Cl
mirror mirror
CH3 pancake CH3 pancake
flip flip
Br Br
I I
B Cl D
Cl
Questions
1. a) Are A and B superimposable?
b) Are A and B mirror images?
c) What is the exact relationship between A and B (one word)?
2. What is the exact relationship between C and D (one word)?
3. a) Are A and C completely superimposable?
b) Are A and C mirror images?
c) What is the exact relationship between A and C (one word)?
4. a) Are A and D completely superimposable?
b) Are A and D mirror images?
c) What is the exact relationship between A and D (one word)?
Model 2: Absolute Configuration and Diastereomers
The absolute configuration of a molecule is its designation as R or S. Review: for molecules with one
stereocenter, 1) one enantiomer will be R and the other will be S, 2) the enantiomer of one molecule
can be drawn as a mirror image or by switching the groups on the dash and wedge of the stereocenter.
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
WS16, CHEM 211, Dr. Poutsma 2
Questions
5. a) Mark each stereocenter in A, B, C, and D with a *.
b) Assign each unassigned stereocenter as R or S.
6. a) Are the carbons in A and B bonded to Br both R, both S or is one R and one S?
b) Are the carbons in A and B bonded to I both R, both S or is one R and one S?
c) What conclusion can you draw about the absolute configurations of enantiomers with multiple
stereocenters?
d) Do your assignments of R and S for C and D (enantiomers) agree with your statement in 6c? If
not, modify your statement to fit the data.
7. a) Are the carbons in A and C bonded to Br both R, both S or is one R and one S?
b) Are the carbons in A and C bonded to I both R, both S or is one R and one S?
c) What conclusion can you draw about the absolute configurations (R and S) of diastereomers?
(Recall that A and C are diastereomers)
d) Do your assignments of R and S for A and D (diastereomers) agree with your statement in 7c?
If not, modify your statement to fit the data.
8. The enantiomer of a molecule with multiple stereocenters can also be drawn two ways. The first
way is to draw the mirror image (A and large B in Model 1). Compare A and the pancake flip of B
(or C and the pancake flip of D) to describe the second way to draw the enantiomer of a molecule
with multiple stereocenters. .
9. Compare A to C and the pancake flip of D to describe a way to draw a diastereomer of a
molecule.
Model 3: The Number of Stereoisomers
A compound has 2n stereoisomers, where n is equal to the number of chiral centers. These
stereoisomers consist of 2n/2 pairs of enantiomers.
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
WS16, CHEM 211, Dr. Poutsma 3
Questions
10. a) How many stereoisomers does a compound with two chiral centers have?
b) How many pairs of enantiomers does this compound have?
c) Do your answers to 10a and 10b agree with the structures given in Model 1? Explain.
11. a) How many stereoisomers does a compound with three chiral centers have?
b) How many pairs of enantiomers does the compound have?
Model 4: Stereoisomers
Conformers – can be interconverted via single bond
rotation (that is, without breaking bonds).
Conformers are alternate representations of the
same molecule.
Constitutional Isomers – have the same molecular
formula but different atom connectivity.
Stereoisomers – have the same molecular formula
AND the same atom connectivity, but CANNOT be
superimposed or interconverted via single bond
rotation.
You cannot buy a bottle of “anti” butane or “gauche” butane because in any sample of butane at
normal temperatures, the molecules are rapidly interconverting among all possible conformations. You
can buy a bottle of cis-butene or trans-butene.
Questions
12. Indicate the relationship between each pair. Choose from stereoisomers, identical, conformers,
constitutional isomers, or different formulas. (Each term is used at least once.)
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
WS16, CHEM 211, Dr. Poutsma 4
End of Class Summary
A molecule with two chiral centers has four possible stereoisomers (R,R; S,S; R,S and S,R). Each
stereoisomer (A) will have one enantiomer (B) and two diastereomers (C & D). For the enantiomers,
both chiral centers will have opposite configuration names (i.e. S,S for A and R,R for B). For disaster-
eomers, only one chiral center will have the opposite configuration name (i.e. S,S for A and S,R for C).
Exercises
1. Identify the following pairs of molecules as identical, constitutional isomers, enantiomers or
diastereomers.
a) OH CH3 b)
H OH OH OH OH OH
HO H
OH CH2CH3
c) Br d)
Br OH OH
H H
HH Br H
H
H H Br
2. Draw structures for the following names.
a) (2R, 3S)-2-amino-3-pentanol
b) (1S, 3S)-1-bromo-3-methylcyclopentane
3. Draw all the possible stereoisomers for the following molecule.
Cl OH
CH3
4. Read sections 5.5, N3.1c and N3.1f in Karty. Recommended problems 5.39, 5.40, 5.46, N3.9 and
N3.17
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
You might also like
- A Course of Mathematics for Engineerings and Scientists: Volume 4From EverandA Course of Mathematics for Engineerings and Scientists: Volume 4No ratings yet
- Stereochemistry LabDocument4 pagesStereochemistry Labmayra perezNo ratings yet
- Problem Set #4: Enantiomers, Diastereomers, and StereochemistryDocument6 pagesProblem Set #4: Enantiomers, Diastereomers, and StereochemistryKarthikeyanNo ratings yet
- Test Bank For Organic Chemistry 3rd Edition Janice SmithDocument36 pagesTest Bank For Organic Chemistry 3rd Edition Janice Smithuprightdrollerjit3t100% (27)
- Full Test Bank For Organic Chemistry 3Rd Edition Janice Smith PDF Docx Full Chapter ChapterDocument36 pagesFull Test Bank For Organic Chemistry 3Rd Edition Janice Smith PDF Docx Full Chapter Chaptersparing.kail.ux9x100% (9)
- Full Download Test Bank For Organic Chemistry 3rd Edition Janice Smith PDF Full ChapterDocument36 pagesFull Download Test Bank For Organic Chemistry 3rd Edition Janice Smith PDF Full Chapterprivitywoolhall.8hvcd100% (21)
- 233 Exam2 B Key Sum19Document8 pages233 Exam2 B Key Sum19Reza KhodadadiNo ratings yet
- Test Bank For Organic Chemistry 3rd Edition Janice SmithDocument15 pagesTest Bank For Organic Chemistry 3rd Edition Janice Smithjacobjasminekpk5No ratings yet
- 3) StereochemistryDocument80 pages3) StereochemistrymijaniallNo ratings yet
- 4.0 Formulas and StructuresDocument21 pages4.0 Formulas and StructuresAj MirandaNo ratings yet
- Supporting LecDocument43 pagesSupporting LecDivyam JainNo ratings yet
- Organic Chemistry by Janice Smith Test BankDocument15 pagesOrganic Chemistry by Janice Smith Test Bankalikaastrid87% (15)
- Isomerism.: 3 Mark QuestionsDocument27 pagesIsomerism.: 3 Mark QuestionsGovindasami RameshNo ratings yet
- Lab Report 6 CHEMDocument9 pagesLab Report 6 CHEMaidaNo ratings yet
- Stereochem Test BankDocument19 pagesStereochem Test BankCarmela Liria100% (15)
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet
- Stereochemistry_uploadDocument51 pagesStereochemistry_uploadjayaramvardhan2No ratings yet
- 6 Tartaric Acid: Erythro Form Threo Form Meso FormDocument13 pages6 Tartaric Acid: Erythro Form Threo Form Meso FormswastikNo ratings yet
- Organic Chemistry IIDocument13 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- Organic Chemistry IIDocument7 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- L3 - Chapter3 - StereochemistryDocument61 pagesL3 - Chapter3 - StereochemistryAbishree GunasekhranNo ratings yet
- StereokimiaDocument32 pagesStereokimiaBrenda GloriaNo ratings yet
- Stereochemistry CHM456Document82 pagesStereochemistry CHM456notmeNo ratings yet
- Organic Chemistry I Homework 2009 Fall Dr. WorkmanDocument15 pagesOrganic Chemistry I Homework 2009 Fall Dr. WorkmanFrancine DinizNo ratings yet
- NCERT Important Chemistry LinesDocument106 pagesNCERT Important Chemistry LinesSridhar ChowdaryNo ratings yet
- ISOMERISM types questionsDocument16 pagesISOMERISM types questionssurendra chowdary Makineni100% (1)
- Stereochemistry Tutorial: Drawing Enantiomers and DiastereomersDocument5 pagesStereochemistry Tutorial: Drawing Enantiomers and DiastereomersacctimNo ratings yet
- PG Organic Unit - IDocument13 pagesPG Organic Unit - IElakkiya shankarNo ratings yet
- Exam #2 PracticeDocument7 pagesExam #2 PracticeNiel Felix Villanueva BalakidNo ratings yet
- CHM3201 Lab Report S2 2019-2020Document42 pagesCHM3201 Lab Report S2 2019-2020Halimatun MustafaNo ratings yet
- 5b Stereochemistry PostDocument41 pages5b Stereochemistry Postapi-3767370100% (1)
- Chapter 5Document41 pagesChapter 5Eshita SharmaNo ratings yet
- Goal 9-1Document8 pagesGoal 9-1Koleti KoletiNo ratings yet
- Worksheet14 HybridDocument5 pagesWorksheet14 HybridRAGINI AGARWALNo ratings yet
- Chapter 3Document60 pagesChapter 3Nahom AmanuelNo ratings yet
- Stereoisomerism advanced level guideDocument5 pagesStereoisomerism advanced level guidearryan keshanNo ratings yet
- Stereochemistry 22Document21 pagesStereochemistry 22Ahmed SideegNo ratings yet
- CH 5. StereochemistryDocument36 pagesCH 5. StereochemistryAhmed ZakyNo ratings yet
- CH 105-CIP RulesDocument16 pagesCH 105-CIP RulesAmitesh ShekharNo ratings yet
- Isomerism: IndexDocument38 pagesIsomerism: IndexDEV UPPALNo ratings yet
- Ebook Chemistry The Molecular Science 5Th Edition Moore Solutions Manual Full Chapter PDFDocument67 pagesEbook Chemistry The Molecular Science 5Th Edition Moore Solutions Manual Full Chapter PDFMeganJonesjwbp100% (9)
- Organic - Class 5 PDFDocument42 pagesOrganic - Class 5 PDFSajan Singh LUCKYNo ratings yet
- Drawing Organic Molecules Thinking About Molecules in 3D Space Naming Alkanes Newman Projections Energy of Conformations Introduction To IsomersDocument21 pagesDrawing Organic Molecules Thinking About Molecules in 3D Space Naming Alkanes Newman Projections Energy of Conformations Introduction To IsomersPigeon BNo ratings yet
- Emm MCQ Unit1Document40 pagesEmm MCQ Unit1Magnus CarlsenNo ratings yet
- Organic Chemistry II: Stereochemistry, Functional Group Compounds, Carbohydrates and MoreDocument58 pagesOrganic Chemistry II: Stereochemistry, Functional Group Compounds, Carbohydrates and Moresanti salsabilaNo ratings yet
- Isomerism DPP - With Solution PDFDocument20 pagesIsomerism DPP - With Solution PDFPiyush Agarwal50% (2)
- Stereochemistry: Concepts For Chiral CompoundsDocument6 pagesStereochemistry: Concepts For Chiral CompoundsSankar AdhikariNo ratings yet
- ICT BHB Sem 2 2Document59 pagesICT BHB Sem 2 2Ayushmaan TripathiNo ratings yet
- Ramachandran Plots. Amino Acid Configuration in ProteinsDocument21 pagesRamachandran Plots. Amino Acid Configuration in Proteinsnancy bhadiarNo ratings yet
- ws13 Stereochemistry Professor Jennifer Poutsma PDFDocument4 pagesws13 Stereochemistry Professor Jennifer Poutsma PDFSankar AdhikariNo ratings yet
- Chem 2016Document4 pagesChem 2016Shubhankar ChakrabortyNo ratings yet
- LG 1.5 Isomerism Part II (Stereoisomerism)Document10 pagesLG 1.5 Isomerism Part II (Stereoisomerism)wangmorisNo ratings yet
- Taller de ESTEREOQUIMICA2021-01Document14 pagesTaller de ESTEREOQUIMICA2021-01alexandra lozadaNo ratings yet
- ISOMERISM - A General Survey: The Carbon SkeletonDocument4 pagesISOMERISM - A General Survey: The Carbon Skeletonzafarchem_iqbalNo ratings yet
- Chem 341 Exam 2 Fall 2017Document15 pagesChem 341 Exam 2 Fall 2017Karmen ReynoldsNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- CSIR NET June 2021 PhysicalDocument45 pagesCSIR NET June 2021 PhysicalSankar AdhikariNo ratings yet
- Schematic representation of numbers on a dieDocument13 pagesSchematic representation of numbers on a dieSankar AdhikariNo ratings yet
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- Quantitative Aptitude Tricks - PDF Download: ImplificationDocument12 pagesQuantitative Aptitude Tricks - PDF Download: ImplificationSwapnarani JadavNo ratings yet
- Master Quantitative Aptitude with tips & tricksDocument24 pagesMaster Quantitative Aptitude with tips & tricksSankar Adhikari0% (1)
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- Common MistakesDocument3 pagesCommon MistakesSankar AdhikariNo ratings yet
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- Synthesis of Vinyl Ketones Via Mannich Bases: Was Nu: (Enolate)Document1 pageSynthesis of Vinyl Ketones Via Mannich Bases: Was Nu: (Enolate)Sankar AdhikariNo ratings yet
- Aromatic TM Homework CHM 4220 Organic Synthesis, Dr. Laurie S. StarkeyDocument1 pageAromatic TM Homework CHM 4220 Organic Synthesis, Dr. Laurie S. StarkeySankar AdhikariNo ratings yet
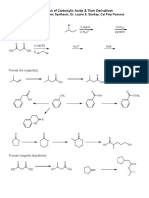
- Preparation of Carboxylic Acids & Their Derivatives: 1) MG 2) Co 3) H O 1) Nacn 2) H O Socl PyridineDocument1 pagePreparation of Carboxylic Acids & Their Derivatives: 1) MG 2) Co 3) H O 1) Nacn 2) H O Socl PyridineSankar AdhikariNo ratings yet
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- Aromatic TM Skeleton NotesDocument7 pagesAromatic TM Skeleton NotesSankar AdhikariNo ratings yet
- Troubleshooting Workup TricksDocument10 pagesTroubleshooting Workup TricksSankar AdhikariNo ratings yet
- Mass Spec NotesDocument6 pagesMass Spec NotesSankar AdhikariNo ratings yet
- IRcorrelation TableDocument1 pageIRcorrelation TableSankar AdhikariNo ratings yet
- Introduction to NMR SpectroscopyDocument1 pageIntroduction to NMR SpectroscopySankar AdhikariNo ratings yet
- Ace General Chemistry 1 and 2Document187 pagesAce General Chemistry 1 and 2Ari Singh100% (2)
- H NMR Problem-Solving StrategiesDocument1 pageH NMR Problem-Solving StrategiesSankar AdhikariNo ratings yet
- Preparation of Ethers & Amines: 1) Nah 2) NaomeDocument1 pagePreparation of Ethers & Amines: 1) Nah 2) NaomeSankar AdhikariNo ratings yet
- Thermochemistry LAB REPORTDocument13 pagesThermochemistry LAB REPORTSolethu MthembuNo ratings yet
- Quimica Organica - Teoria AtomicaDocument13 pagesQuimica Organica - Teoria AtomicaManuelNo ratings yet
- Frising 2006Document24 pagesFrising 2006samandondonNo ratings yet
- Alcohol Ether and ExpoksideDocument64 pagesAlcohol Ether and ExpoksideAhmadBadruzzamanShuib100% (1)
- Fiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Document9 pagesFiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Lokesh KumarNo ratings yet
- Computational Chemistry Unit IDocument36 pagesComputational Chemistry Unit ITesfamariam Setargew MesfinNo ratings yet
- Electrochemical Cells ExplainedDocument27 pagesElectrochemical Cells ExplainedKazim RazaNo ratings yet
- Optical Micros PDFDocument24 pagesOptical Micros PDFsensoham03No ratings yet
- Chemical ReactionDocument30 pagesChemical ReactionAnna Liza GomezNo ratings yet
- Logic Puzzle ChemistryDocument6 pagesLogic Puzzle ChemistryBarnali DuttaNo ratings yet
- General Chemistry I - Tutorial 5Document6 pagesGeneral Chemistry I - Tutorial 5Duc Anh NguyenNo ratings yet
- جهاز قياس الناقلية - EnglishDocument13 pagesجهاز قياس الناقلية - EnglishAhmad A ShamiNo ratings yet
- Acumer3100 Cooling PDFDocument7 pagesAcumer3100 Cooling PDFdalton2003No ratings yet
- Synthesis of Fatty Alcohols from Oil Palm Using Ni-Cu Catalyst on ZeoliteDocument18 pagesSynthesis of Fatty Alcohols from Oil Palm Using Ni-Cu Catalyst on ZeoliteHelmi BaharNo ratings yet
- Inf Ufc 85Document13 pagesInf Ufc 85Luciano Montellano Abasto100% (2)
- Lab 1 107 Freezing Point Depression and Ice CreamDocument6 pagesLab 1 107 Freezing Point Depression and Ice CreamnasuhaNo ratings yet
- Chemistry Unit 1 Revision 1Document23 pagesChemistry Unit 1 Revision 1cuchikapoorNo ratings yet
- AnswersDocument3 pagesAnswersapi-240032800No ratings yet
- CH 8 and 10 - Basic Principles of Atomic Absorption and Atomic Emission SpectrosDocument129 pagesCH 8 and 10 - Basic Principles of Atomic Absorption and Atomic Emission SpectrosfaisalNo ratings yet
- Comparison of Hydrogen Specification in National Standards For ChinaDocument5 pagesComparison of Hydrogen Specification in National Standards For Chinabarun1977No ratings yet
- Chap 1 Periodic Table ExerciseDocument26 pagesChap 1 Periodic Table ExerciseAbhimanyu GuptaNo ratings yet
- CHE505 ChapterTwo Multiphase Reactor PDFDocument40 pagesCHE505 ChapterTwo Multiphase Reactor PDFShammil AshmanNo ratings yet
- Chapter 7 ColloidsDocument31 pagesChapter 7 ColloidsAshelNo ratings yet
- Chemisorption Chiller Performance with Heat and Mass RecoveryDocument9 pagesChemisorption Chiller Performance with Heat and Mass RecoveryOcta RioNo ratings yet
- EPA Study GuideDocument58 pagesEPA Study GuideChand Ramnarain67% (3)
- Question Bank For First Year First Sem Question Bank For Physics-I Regulation 20913Document21 pagesQuestion Bank For First Year First Sem Question Bank For Physics-I Regulation 20913PRIYA RAJINo ratings yet
- 1-Adsorption FINAL PDFDocument41 pages1-Adsorption FINAL PDFFransiscaa HellenNo ratings yet
- Solute and SolventDocument12 pagesSolute and SolventuminoriahNo ratings yet
- ME 424 Refrigeration Systems GuideDocument10 pagesME 424 Refrigeration Systems GuideJustin MercadoNo ratings yet
- I-P-1.01-W-1 (CO Analyser)Document2 pagesI-P-1.01-W-1 (CO Analyser)Mechanical ShauryaNo ratings yet