Professional Documents
Culture Documents
1.3 Lipids
Uploaded by
BlitzSZN0 ratings0% found this document useful (0 votes)
7 views1 pageThis document discusses ester bonds and lipids. It summarizes that:
1) Ester bonds are formed from a condensation reaction between one glycerol molecule and three fatty acid chains, providing a good source of energy in a small volume due to many C-H bonds.
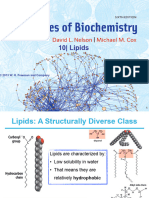
2) Triglycerides are insoluble in water and made from one glycerol molecule, two fatty acid chains, and one phosphate molecule.
3) Phospholipids have a hydrophilic "head" and hydrophobic "tail" and are a component of cell membranes, forming a phospholipid bilayer.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses ester bonds and lipids. It summarizes that:
1) Ester bonds are formed from a condensation reaction between one glycerol molecule and three fatty acid chains, providing a good source of energy in a small volume due to many C-H bonds.
2) Triglycerides are insoluble in water and made from one glycerol molecule, two fatty acid chains, and one phosphate molecule.
3) Phospholipids have a hydrophilic "head" and hydrophobic "tail" and are a component of cell membranes, forming a phospholipid bilayer.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views1 page1.3 Lipids
Uploaded by
BlitzSZNThis document discusses ester bonds and lipids. It summarizes that:
1) Ester bonds are formed from a condensation reaction between one glycerol molecule and three fatty acid chains, providing a good source of energy in a small volume due to many C-H bonds.
2) Triglycerides are insoluble in water and made from one glycerol molecule, two fatty acid chains, and one phosphate molecule.
3) Phospholipids have a hydrophilic "head" and hydrophobic "tail" and are a component of cell membranes, forming a phospholipid bilayer.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Ester bonds
Lots of energy in a Made from 1 glycerol
small volume molecule and 3 fatty Condensation
acid chains reaction
Many C-H bonds = lots Good source of
of energy energy Made from 1 glycerol
molecule, 2 fatty acid
Insoluble in chains and 1 phosphate
No effect on osmosis Triglycerides molecule
in cells water
Tests Hydrophilic
Cloudy-white colour = 1.3 LIPIDS Phospholipids ‘head’,
positive result hydrophobic ‘tail’
Basis of test - Emulsion test - Polar
lipids emulsify add ethanol and Monomers Component of cell
in the water water, shake membrane -
and form tiny
phospholipid
droplets Fatty acids have
bilayer ‘Heads’ face towards
the formula aqueous environments
Insoluble in water, Fatty acids and RCOOH e.g. cytoplasm
soluble in organic glycerol are the
solvents (e.g. monomers of lipids Fatty acids can be unsaturated
alcohol) (contain a C=C bond) or saturated
(all C-C bonds) AQA
https://bit.ly/pmt-cc
https://bit.ly/pmt-cc
You might also like
- Food SafeDocument230 pagesFood Safenayadeth22No ratings yet
- FRP Catalogue NewDocument82 pagesFRP Catalogue Newamol ganvirNo ratings yet
- Skincare Formulation CC Cream Asian SkinDocument2 pagesSkincare Formulation CC Cream Asian SkinKhair ki BateyNo ratings yet
- Exam 1 AnswersDocument9 pagesExam 1 AnswersArvic Lacson0% (1)
- Handbook of Industrial Water Treatment @14.2.2022Document401 pagesHandbook of Industrial Water Treatment @14.2.2022Naung100% (1)
- Nanotechnology For Water TreatmentDocument19 pagesNanotechnology For Water TreatmentCHIEF VISHAAL 45100% (1)
- ASTM D 512 Standard Test Methods For Chloride Ion in Water PDFDocument7 pagesASTM D 512 Standard Test Methods For Chloride Ion in Water PDFBilalNo ratings yet
- Science7 - q1 - Mod4 - I Have Less, She Has Ample, He Has More, Let Us See What - S in Store - FINAL08032020Document27 pagesScience7 - q1 - Mod4 - I Have Less, She Has Ample, He Has More, Let Us See What - S in Store - FINAL08032020ruff92% (24)
- Control of Vapor Recovery Units (VRU)Document8 pagesControl of Vapor Recovery Units (VRU)Yasmine ياسمينNo ratings yet
- Biología 2Document3 pagesBiología 2María PNo ratings yet
- Chem123lec MidtermDocument18 pagesChem123lec Midtermlily of the valleyNo ratings yet
- 3.2 Carbohydrates Lipids Proteins WORDDocument6 pages3.2 Carbohydrates Lipids Proteins WORDCaitlin Barrett100% (1)
- 3.5 Lipids - A Level BiologyDocument3 pages3.5 Lipids - A Level BiologyElliott MasonNo ratings yet
- Unit 3. LipidsDocument33 pagesUnit 3. LipidsHBJBHNo ratings yet
- Aqa A Level Biology Theory v1Document38 pagesAqa A Level Biology Theory v1Thein KoNo ratings yet
- Biochemistry LipidsDocument17 pagesBiochemistry LipidsMohammed EljackNo ratings yet
- (BIOCHEM LAB) LipidsDocument8 pages(BIOCHEM LAB) Lipidsprettyfriends 05No ratings yet
- Bio 2Document14 pagesBio 2Maria Jeorgia SalinasNo ratings yet
- Nomenclature:: Example: Docosahexaenoic acid/DHA (LinoleicDocument4 pagesNomenclature:: Example: Docosahexaenoic acid/DHA (Linoleicgizelle mae pasiolNo ratings yet
- Module 2 BiochemDocument13 pagesModule 2 BiochemAbby Dimalaluan OquendoNo ratings yet
- B. LipidsDocument11 pagesB. LipidsMary Rose Bobis VicenteNo ratings yet
- K01299 - 20191111164222 - SBL 1023 Lec 8Document54 pagesK01299 - 20191111164222 - SBL 1023 Lec 8Alia HanisaNo ratings yet
- Biochemistry Week 8 - LipidsDocument7 pagesBiochemistry Week 8 - LipidsMicah JadeNo ratings yet
- Chem 123 - LipidsDocument11 pagesChem 123 - LipidsGylene GardonNo ratings yet
- Characterization of LipidsDocument4 pagesCharacterization of LipidsJustin VillanuevaNo ratings yet
- Lipids PT 1 Lipids?: StorageDocument14 pagesLipids PT 1 Lipids?: StorageRegil Mae GaleroNo ratings yet
- LipidsDocument14 pagesLipidsBeverlyNo ratings yet
- LipidsDocument6 pagesLipidsJohn Fritz Gerald BascoNo ratings yet
- (Biochem A) Lipid Chemistry-Alcantara (Gradelifting Fairies)Document11 pages(Biochem A) Lipid Chemistry-Alcantara (Gradelifting Fairies)bero beroNo ratings yet
- Bio MoleculesDocument77 pagesBio MoleculesDeepti NageshNo ratings yet
- LipidsDocument7 pagesLipidsHana LunariaNo ratings yet
- Chapter 4 Biology Form 4Document34 pagesChapter 4 Biology Form 4nuriNo ratings yet
- LipidsDocument4 pagesLipidsKriszel Joy MaderiaNo ratings yet
- Lipids NotesDocument17 pagesLipids Notescsdeguzman7241pamNo ratings yet
- Lipids: o Dehydration Synthesis Is When TheDocument3 pagesLipids: o Dehydration Synthesis Is When TheKheza SuravillaNo ratings yet
- Organic Compounds: OutlineDocument4 pagesOrganic Compounds: Outlinevinnie0905No ratings yet
- Notes On LipidsDocument4 pagesNotes On LipidsCheyenne Kaye EspirituNo ratings yet
- Insoluble in Water, But Soluble in Nonpolar Solvents and Solvents of Low PolarityDocument5 pagesInsoluble in Water, But Soluble in Nonpolar Solvents and Solvents of Low Polarity3amabelle arevaloNo ratings yet
- Insoluble in Water, But Soluble in Nonpolar Solvents and Solvents of Low PolarityDocument5 pagesInsoluble in Water, But Soluble in Nonpolar Solvents and Solvents of Low Polarity3amabelle arevaloNo ratings yet
- FATS and LIPIDSDocument2 pagesFATS and LIPIDSAlyssa LumaadNo ratings yet
- Examination of LipidsDocument3 pagesExamination of LipidsJannah IsmaelNo ratings yet
- Ruphele Anne I. QuitagDocument4 pagesRuphele Anne I. QuitagCiara marie BernardoNo ratings yet
- Lipids-And-Lpp CCDocument11 pagesLipids-And-Lpp CCnamormarie10No ratings yet
- LipidsDocument26 pagesLipidsZanie CruzNo ratings yet
- Lipids 2Document132 pagesLipids 2jasonenmanuel10No ratings yet
- Lipids Part IDocument5 pagesLipids Part IMar LagmayNo ratings yet
- 2.2 Macromolecules HomeworkDocument4 pages2.2 Macromolecules HomeworkKarolina RachwalNo ratings yet
- LipidDocument36 pagesLipidSina AgataNo ratings yet
- LipidsDocument4 pagesLipidsshane.surigaoNo ratings yet
- Biochemistry I BSC 211: LipidsDocument16 pagesBiochemistry I BSC 211: LipidsKelvin ChipezeniNo ratings yet
- Chapter V: LIPIDSDocument23 pagesChapter V: LIPIDSGwyneth Marie DayaganNo ratings yet
- 03 Lipids StudentsDocument40 pages03 Lipids Studentsmakabigail7No ratings yet
- LG 4 Digestion and Human HealthDocument148 pagesLG 4 Digestion and Human Health86t8gx9y8zNo ratings yet
- Biological Molecules: When X y It's Simple So CX (H20) y When X Does Not y It's Simple So CX (H20) yDocument15 pagesBiological Molecules: When X y It's Simple So CX (H20) y When X Does Not y It's Simple So CX (H20) yteeeNo ratings yet
- 10 - Lipids: © 2013 W. H. Freeman and CompanyDocument71 pages10 - Lipids: © 2013 W. H. Freeman and Companymyatpwintp880No ratings yet
- Unit-14 Biomolecules Mini 2023Document5 pagesUnit-14 Biomolecules Mini 2023jagannathanNo ratings yet
- Carbohydrates, Lipids, Proteins and Nucleic AcidsDocument49 pagesCarbohydrates, Lipids, Proteins and Nucleic Acidssos229072100% (1)
- Biochem Mod 9 PDFDocument5 pagesBiochem Mod 9 PDFtheaNo ratings yet
- M2 Lipids: Biochem LecDocument7 pagesM2 Lipids: Biochem LechaafizaNo ratings yet
- Lipase LittlelaptopDocument53 pagesLipase LittlelaptopAjay LakshmananNo ratings yet
- Lipids: Lipids Known As Fats Provide A MajorDocument82 pagesLipids: Lipids Known As Fats Provide A MajorYoshiNo ratings yet
- 2.1 Lipid Chemistry Part 1Document6 pages2.1 Lipid Chemistry Part 1Alexandra DalisayNo ratings yet
- Learning Outcomes: 1.3 LipidsDocument29 pagesLearning Outcomes: 1.3 LipidsJack Si Yi WeiNo ratings yet
- Lipids I-Iv: Prepared By: Group 6Document11 pagesLipids I-Iv: Prepared By: Group 6Jan Edward Abarientos MandaniNo ratings yet
- Lipids I-Iv: Prepared By: Group 6Document11 pagesLipids I-Iv: Prepared By: Group 6Jan Edward Abarientos MandaniNo ratings yet
- LipidDocument49 pagesLipidLe Thanh HaiNo ratings yet
- Condensation Hydrolysis2Document13 pagesCondensation Hydrolysis2EmNo ratings yet
- B. Glycerol: Classes and Properties of Simple LipidsDocument3 pagesB. Glycerol: Classes and Properties of Simple LipidsTRISHA MARIE CLEMENTENo ratings yet
- Free Radical Substitution Questions: Ex. Methane + ChlorineDocument2 pagesFree Radical Substitution Questions: Ex. Methane + ChlorineVimal AathithanNo ratings yet
- Experiment 1-Emulsion Polymerization of PolystyreneDocument3 pagesExperiment 1-Emulsion Polymerization of Polystyrenekarimakar684No ratings yet
- Preparation, Characterization and Antimicrobial Activity of Chitosan - ZN ComplexDocument6 pagesPreparation, Characterization and Antimicrobial Activity of Chitosan - ZN ComplexFernanda Stuani PereiraNo ratings yet
- Design of Boiler Forced Draft FanDocument5 pagesDesign of Boiler Forced Draft FanAu TagolimotNo ratings yet
- Liquid or Dye Penetrant InspectionDocument43 pagesLiquid or Dye Penetrant InspectionTrinadh Venkata Kumar NillaNo ratings yet
- Method For Nitrate Determination by Cadmium Reduction, Version 2.3Document8 pagesMethod For Nitrate Determination by Cadmium Reduction, Version 2.3KhalidOfqirNo ratings yet
- Supergene Metal DepositsDocument6 pagesSupergene Metal DepositsRalph Carlo EvidenteNo ratings yet
- G 02 01 02 Specification Fuel OilDocument1 pageG 02 01 02 Specification Fuel OilJahurul IslamNo ratings yet
- New Jss2 3rd Term E-Learning Notes Revised 2017Document14 pagesNew Jss2 3rd Term E-Learning Notes Revised 2017EMMA EMOLENo ratings yet
- 'TM-Ring Test' - A Quality Control Test For TMT (Or QST) Steel Reinforcing Bars Used in Reinforced Concrete SystemsDocument10 pages'TM-Ring Test' - A Quality Control Test For TMT (Or QST) Steel Reinforcing Bars Used in Reinforced Concrete SystemsAkankwasa RonaldNo ratings yet
- MSDS - Bitumen Rev1Document4 pagesMSDS - Bitumen Rev1benzeneinternationalNo ratings yet
- Some BasicDocument112 pagesSome BasicAditya BansalNo ratings yet
- 6.metabolisme Lipid PDFDocument40 pages6.metabolisme Lipid PDFSYITA SHEVIA PRASASTINo ratings yet
- Aqua Chemicals FreshwaterDocument7 pagesAqua Chemicals FreshwaterJuthi RahmanNo ratings yet
- Assessment of Ground Water Quality in and Around Gobichettipalayam Town Erode District, TamilnaduDocument6 pagesAssessment of Ground Water Quality in and Around Gobichettipalayam Town Erode District, TamilnadufosterbalaNo ratings yet
- Clin-Tech Microbiology BrochureDocument10 pagesClin-Tech Microbiology Brochurehoria96No ratings yet
- Aalco Metals LTD - Stainless Steel 14016 430 Sheet and Plate - 95Document3 pagesAalco Metals LTD - Stainless Steel 14016 430 Sheet and Plate - 95bhushansalunkeNo ratings yet
- Welded Wire Mesh SolutionsDocument12 pagesWelded Wire Mesh SolutionsDilhara WickramaarachchiNo ratings yet
- Synthesis of Asprin Lab ReportDocument3 pagesSynthesis of Asprin Lab ReportRachelleNo ratings yet
- Pang 2003Document8 pagesPang 2003Marius FlorinNo ratings yet