Professional Documents
Culture Documents
Pacemakers PK2 Jan2018
Uploaded by
ItachiCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pacemakers PK2 Jan2018
Uploaded by
ItachiCopyright:
Available Formats
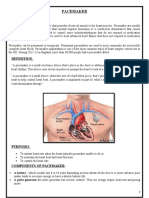
The Microtechnology of Pacemakers
Primary Knowledge
Unit Description
In the unit – The Heart and Pacemakers – you learned how the heart works, problems with the
heart that can affect its rhythm, and when pacemakers are needed to monitor and correct heart
rhythm. In this unit you explore the microtechnology of today’s pacemakers and how this
technology is being used to improve lives. You learn about the electronics and the mechanics of
pacemaker operation, how MEMS (microelectromechanical systems) are used to execute these
operations, and how pacemakers are used to sense changes in a patient’s heart rhythm and
activity and compensate for these changes.
Estimated Time to Complete
Allow about 30 minutes to study this material.
Introduction
Artificial pacemakers are medical devices that are
used to help regulate one’s heart rate due to abnormal
heart rhythms (arrhythmias) or heart block - problems
with the heart’s natural electrical system. Pacemakers
consist of a pulse generator and one to three
electrodes that are fed from the generator to specific
locations of the heart.
Pacemaker: Pulse Generator with single electrode
[Images courtesy of Stevernfruitsmaak via Creative Commons]
The pulse generator contains the microprocessor and sensors. When the heart needs assistance,
an electrical pulse is sent from the generator to the heart muscle via the electrodes, stimulating
the heart muscle and creating a contraction. The electrical pulse is generated when the
generator’s sensors sense that a pulse is needed.
Defibrillators generate an intermittent pulse or a pulse as needed or “on demand”. Pacemakers,
or more specifically, “rate responsive” pacemakers generate continuous pulses, beating the heart
100% of the time. MEMS (microelectromechanical systems) sensors and other sensors inside the
generator sense changes in the patient’s movements, such as walking or sitting, and adjust the
heart’s “rate” or beats per minute to meet the activity (i.e., a faster heart rate when walking than
when sitting).
Southwest Center for Microsystems Education (SCME) Page 1
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
Defibrillator and Pacemaker
[Image courtesy of National Heart Lung and Blood Institute – National Institute of Health]
As mentioned in the previous unit (The Heart and Pacemakers), pacemakers are implantable
medical devices, meaning that the electrodes and the generator are in vivo or inside the body.
The generator is usually placed below a collarbone just under the skin. The generator’s
electrodes are inserted into a vein leading to the heart. Depending on the number of electrodes
needed, electrodes may be placed in the right ventricle, the right atrium, or both. When a third
electrode is needed, it is fed through a vein on the surface of the heart and connected to the left
ventricle.
This unit covers the following topics:
Pacemaker components
MEMS Sensors and Components
Pacemaker electrodes
A brief pacemaker history
Learning Module Objectives
Explain how MEMS accelerometers monitor and control heart rhythm in pacemaker
patients.
Outline how a rate responsive pacemaker works.
Identify the limitations of current pacemakers and recommend ways to eliminate
these limitations in future pacemakers.
Southwest Center for Microsystems Education (SCME) Page 2
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
Pacemaker Components
Pacemaker refers to the complete device consisting of the pulse generator and the electrodes.
The Pulse Generator
The pulse generator does much more than just generate a pulse. The pulse generator contains
the control and sensing units of a pacemaker as well as data storage. Essentially, it is a small
computer hermetically sealed in a titanium case. Titanium is used because it is ten times as
strong as steel and lighter in weight, is resistant to corrosion, and extremely durable. But most
importantly, it is biocompatible, making it an excellent material for pacemaker casings.8
What is inside the pulse generator of a pacemaker?
Lithium-Iodine Battery
Circuit board
o Microprocessor with circuitry, clock,
and data storage
o MEMS Sensors
o Reed Sensor
Electrode Connections
A pacemaker’s battery takes up about half of the
space inside the case and is the only power source
for all of the electrical components (microprocessor,
data storage, sensors, electrodes); therefore, it must
meet some very stringent criteria8:
Lightweight and flat (usually weighs
between 12.5 grams to 15.5 grams) A peek inside the pulse generator,
Highly reliable electrode connections, and electrodes
Accurate (minimum/maximum voltages; initial,
average, maximum discharge current)
Continuous and/or intermittent operation
Long shelf life
Long and predictable service life
Impact resistant
Perform in a variety of conditions (changing temperatures, duty cycle, vibrations, etc.)
The typical output voltages of a pacemaker battery are 1.5 to 6.0 volts and a typical battery
capacity of 1 amp-hour. For a general reference, a typical lead-acid car battery has a capacity of
100 amp-hours at 12 volts.9
Today’s pacemaker batteries are designed to last between 8 to 12 years, depending on the
manufacturer, the amount of power required by the pulse generator, and the amount of pulsing
required by the patient. For example, a rate responsive pacemaker with two electrodes working
24/7, normally has a shorter life than an on-demand generator that sends out a pulse only as
needed.
Southwest Center for Microsystems Education (SCME) Page 3
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
The battery life is checked during normal pacemaker checkups, two to four times a year. During
a battery’s life, about half of its energy is used to pulse the heart, while the other half is used to
supply power to the pacemaker electronics and devices. During a check-up, the life of the
battery is checked. Once the battery shows it has less than three months of charge left, the pulse
generator is replaced. The new pulse generator is implanted during a relatively simple, out
patient surgery where the old generator is disconnected from the existing electrodes and replaced
with the new generator. The electrodes are normally not replaced.
Present research is working toward smaller batteries that can supply more current and that last
longer than the batteries used today. Two such batteries include the lithium carbon monoflouride
battery and the lithium polycarbon fluoride battery. There has also been quite a bit of progress
made on “energy harvesters”, batteries that can recharge by harvesting the energy generated by a
patient’s movement and a small magnetic field.10 Once such batteries can be successfully used
for pacemakers, generator replacement due to low battery may become obsolete.
Diagram of the interactions between components on a pacemaker’s circuit board
The microprocessor is the brain of the pacemaker. Like all microprocessors, it controls the
“action” of the pacemaker. It receives the outputs from the MEMS sensing devices and other
sensors, interprets the data, and responds as programmed. Every action that is performed and
even some actions that are not performed are recorded and stored. Voltage levels, current levels,
events, times, heart rates, missed contractions, fibrillations, durations, frequencies – all of this
data and more are measured, monitored and recorded by the microprocessor. On a regular basis,
this data is collected via a visit by the patient to the pacemaker clinic, or a data dump over a
phone line or WiFi.
Software determines “how” the generator responds to certain situations. When a pacemaker is
initially implanted, the software is programmed according to the known needs of the patient.
This software is modified as the needs of the patient change or to better suit the needs of the
patient. For example, some patients may find that the sensitivity of the MEMS sensors to be too
Southwest Center for Microsystems Education (SCME) Page 4
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
sensitive (e.g., increases the heart rate too much with a slight increase in activity) or not sensitive
enough (e.g., increases the heart rate too slowly or not enough with an increase in activity). A
quick visit to the pacemaker clinic corrects this using an external programmer that “talks”
wirelessly to the pacemaker program and makes the necessary adjustments.
MEMS Sensors and Components
The MEMS sensor most commonly used in
rate responsive pacemakers is the 3-axis
MEMS accelerometer sensor, a type of
initial sensor consisting of three micro-sized
accelerometers as shown in the image to the
right. These accelerometers measure
movement in the x, y and z directions in a 3-
dimensional space. This 3-axis
accelerometer is the same type of sensor
used in smart phones and tablets,
automobiles, seismographs, and engineering
and medical equipment that measure
vibrations, movement, rotation and
acceleration. The package size for this
STMicroelectronics 3-axis accelerometer to
the right is 3 mm x 3 mm.15 STMicroelectronics 3-axis accelerometer
SEM (Scanning Electron Microscope) image
[Image courtesy of Chipworks15]
A 3-axis accelerometer sensor consists of two “in-plane” accelerometers (x and y) and one “out-
of-plane” accelerometer (z). In the SEM above, the two in-plane accelerometers are shown side-
by-side at the top left of the image. These are the X-Y sensors. The out-of-plane accelerometer
is in the center. This is the Z sensor.
In-Plane Both types of accelerometers work by
accelerometer sensing a minute change in capacitance
between electrodes as the fingers move
relative to each other. The image to the
left is an in-plane accelerometer. This
type of accelerometer contains sets of
electrode “fingers” shown here in red,
blue and orange. The red and orange
electrodes are fixed or stationary while
the blue electrodes are allowed to move
up and down due to microfabricated
“springs” at both ends. When there is
movement in the plane of the electrodes, the blue fingers shift up or down in the plane, causing a
change in capacitance between the multiple electrode fingers. This change is interpreted by the
microprocessor as “activity” and the amount of change is interpreted as a proportional change in
activity. A change in the heart rate is generated based on the amount of changed sensed.
Southwest Center for Microsystems Education (SCME) Page 5
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
The out-of-plane accelerometer, shown to the right
in a scanning electron microscope image (SEM),
uses a moveable mass as one electrode and the
substrate as the fixed electrode. When there is
movement in the z direction, the mass moves up out
of its plane enabled by the fabricated micro-springs.
This movement causes a change in capacitance
between the two electrodes. This change is
interpreted by the microprocessor as “activity” and
again, the amount of change is interpreted as a
proportional change in activity, resulting in a
change in heart rate.
[Image courtesy of Khalil Najafi, University of Michigan]
This 3-axis accelerometer sensor is one of the
“packages” mounted on the pulse generator’s
microprocessor board. The accelerometer sensor
interfaces with the microprocessor relaying minute
changes in capacitance proportional to any movement
or activity sensed by the individual accelerometers. An
increase or decrease in activity results in an increase or
decrease to the heart rate, respectively. For example,
when a pacemaker patient goes from a walk to a run,
this increase in activity is immediately sensed by the
accelerometer sensor that generates an output to the
microprocessor that increases the rate of the electronic
pulses being sent to the electrodes. This increase in
electronic pulses results in a faster heat rate needed for
running. As the patient’s pace slows down, the Pacemaker “packages” in blue
accelerometer sensor’s output changes, causing a mounted on circuit board (green)
decrease in the rate of electronic pulses to the heart and
thus a lower heart rate.
In rate responsive pacemakers the accelerometer sensor is the primary activity sensor because of
its ability to adapt quickly and effectively to changes in exercise and other movements. The
characteristics of the accelerometer’s response to changes in activity are adjusted as needed
within the software. For example, some pacemaker patients may require a higher heart rate or a
faster response rate than others for the same activity or movement; therefore, a slight adjustment
to the program is made in order to achieve the desired adaptive rate response from the
accelerometers. A trained pacemaker technician makes such adjustments wirelessly.
One of the disadvantages of accelerometer sensors is their inability to adequately respond to
activity not related to changes in acceleration, vibration, or distinct movements. Such activities
include isometric exercises, physiological stress, picking up and carrying heavy loads, metabolic
Southwest Center for Microsystems Education (SCME) Page 6
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
inadequacy due to pathologic conditions, and even riding a bike. Accelerometer sensors have
also been found to be inadequate for longer exercise sessions when the activity or movement
remains constant but the oxygen debt (or requirement) of the patient increases.11
To address these inadequacies, another sensor can be used as a complementary sensor to the
MEMS accelerometers. This sensor is the minute ventilation sensor, a type of metabolic sensor.
Minute ventilation sensors are used to better solve the problems associated with chronotropic
incompetence (the inability of the SA node to react adequately to exercise and other movement),
isometric exercise and physiological stress. Such sensors have the ability to detect an increase in
oxygen consumption due to factors that do not involve vibrations, accelerations, or other
physical movements.
For example, have you ever experienced an increase in heart rate due to “almost” having a car
accident? The near miss of a tragic accident triggers your sympathetic nervous system (SNS)
causing an increase in heart rate for the purpose of supplying your body with enough oxygenated
blood to remain functional until the stressful situation subsides. Many pacemaker patients do not
experience an increased heart rate in such situations but do experience the stress. Their bodies
call for more oxygen due to the increased stress, but because the heart rate doesn’t increase on its
own or as a result of the accelerometer sensor, these patients can suffer additional stress due to
lack of needed oxygen. Minute ventilation sensors help to address such problems.
Minute ventilation is the product of respiratory rate (the number of breaths per minute) and tidal
volume (the lung volume representing the amount of air inhaled and exhaled during normal,
relaxed breathing). Minute ventilation correlates directly with oxygen consumption, cardiac
output (CO = heart rate x stroke volume), and heart rate (bpm).12
Minute ventilation = respiratory rate x tidal volume = RR x TV
The minute ventilation sensor in a pacemaker “measures transthoracic impedance (the body’s
resistance to current flow) between the electrode tip of a standard bipolar pacemaker lead and the
pulse generator case.” The sensor “generates periodic, low-amplitude electric pulses that
identify changes in thoracic impedance during breathing”13. This change in thoracic impedance
is sensed by the change in electrode current, which is monitored by circuitry in the pulse
generator. As the impedance increases, the electrode current decreases indicating a need to a
faster heart rate.
An example of when a minute ventilation sensor controls heart rate is when you are under
extreme stress. When stressed you might start to breathe heavier, taking in larger volumes of air,
or you might start to breathe faster. Each of these changes increases your transthoracic
impedance. This increase in impedance changes the minute ventilation calculation thus
indicating that a change in heart rate is needed. An increase in impedance results in an increase
in the pacing rate or heart rate, while a decrease in impedance results in a decrease in heart rate.
It has been found that the increases in heart rate as determine by minute ventilation sensors
“closely parallel physiologic variables of metabolic demand.” In other words, these sensors react
in much the same manner as a person without a pacemaker would react under physiologic
stress.14
Southwest Center for Microsystems Education (SCME) Page 7
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
The following graph illustrates “Minute Ventilation and Pacing Rate relative to time”.11 As you
can see, the pacing rate lags slightly behind changes in minute ventilation, but the response is
mostly parallel.
Minute Ventilation and Pacing Rate relative to time.11
Transthoracic impedance can differ from individual to individual due to differences in body
mass, age, disease, and skin resistance. It also fluctuates with physiological changes due to a
variety of exercises, body movements, and mental and physical stress. Therefore, like the
accelerometer sensor, the programming for minute ventilation sensors needs to be adjusted for
each specific patient.
Where else does the minute ventilation sensor work when the accelerometer does not?
Let’s take the situation of a patient walking in a hilly area. The activity of walking is sensed by
the accelerometers, and as this activity increases, so does the heart rate. But what happens as the
person starts walking uphill? In many cases the pace remains the same but the need for oxygen
increases because the work rate increases. The patient starts to breathe faster or takes in a larger
volume of air in an attempt to get more oxygen. In other cases the walking pace may actually
slow down as the patient heads uphill, but the demand for oxygen remains the same or increases.
I’m sure you have sensed this when going uphill or walking up a flight of stairs. The
accelerometer sensor does not sense the need for more oxygen, therefore, it responds to a
decrease in activity with a decrease in the patient’s heart rate. This is when the minute ventilation
sensor kicks in.
As the patient heads up the hill, the respiratory rate and/or volume of inhaled air changes because
the body has a greater demand for oxygen. This change causes a change in thoracic impedance
that is sensed by the minute ventilation sensor and sent to the microprocessor. The
microprocessor calculates the minute ventilation and adjusts the electronic pulses to the
electrodes as needed thus changing the heart rate to better handle the activity.
Southwest Center for Microsystems Education (SCME) Page 8
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
Reed Sensor
Previously we mentioned that the pacemaker’s software is constantly collecting data and storing
it in programmable memory on the pulse generator’s circuit board. So how does this data get to
the doctor and the patient? All of this data can be transmitted and downloaded by creating a data
transfer path between the programmable memory and an external computer. A reed sensor
inside the pulse generator creates this path.
A reed sensor is a device that contains a reed switch but has additional intelligent circuitry and
can withstand higher shock than a discrete switch. The reed switch is an electronic switch that
opens and closes based on the absence or presence of a magnetic field. Reed switches consist of
two contacts on ferromagnetic reeds in a hermetically sealed glass tube (left image below). The
switch is normally open, preventing the flow of current or thus data. However, when a strong
enough magnetic field comes within close proximity of the switch, the reeds close (image right),
allowing for the flow of current and thus the transmission of data.
Reed Sensor – Open Position Reed Sensor – Closed Position
As previously mentioned, a pacemaker technician using an external programming device can
download data, reprogram the pacemaker, and make adjustments wirelessly. In order to do this,
the technician places a magnet on or close to the patient’s chest, directly over the generator
causing the reed switch inside the generator to close. This creates the path for data transfer.
Advantages to using reed sensors are that they are hermetically sealed in the glass tube, free from
contamination, and are immune to electrostatic discharge (ESD). They can also withstand higher
shock than the discrete switches, which is ideal for pacemakers.
Most pacemakers currently use discrete reed sensors mounted on the printed circuit board (PCB).
However, like with many technologies, things are getting smaller. Because discrete reed sensors
are 3-5 millimeters in length, they take up quite a bit of real estate relative to the overall size of
Southwest Center for Microsystems Education (SCME) Page 9
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
the generator’s circuit board. Therefore, in order to make the pulse generators smaller, it would
make sense to make reed sensors and other PCB components smaller, or even better, to
integrated the discrete components in the integrated circuitry.
The current world wide market value for all types of reed switches is around $900 million and
represents a diverse set of customers – medical, automotive, and industrial controls, just to name
a few.19 With such a huge, diverse market, it would make sense that reed switches 1 mm and
smaller and with negligible power consumption would be desirable.
Back in 2007 two companies, HT Micro and Coto Technology started working together to design
and develop MEMS-based reed switches. Both companies have succeeded in this effort. In fact,
in February of 2015, Coto Technology announced the release of the “World’s smallest MEMS-
based magnetic reed sensor…”.16 This reed sensor is in a package of only 1.16 mm3 (smaller
than a grain of rice) and offers directional magnetic sensitivity and ESD (electrostatic discharge)
resistance “while consuming zero power”! These characteristics make it ideal for “emerging
medical device applications, including ingestibles and implantables.” One of the characteristics
of this sensor that makes it quite appealing to pacemakers and other medical devices is its
directional magnetic sensitivity. “Directional sensitivity reduces the risk of false triggers in
sensing applications.”16
Pacemaker Leads or Electrodes
Pacemaker leads or electrodes are ultra-thin, insulated wires
that carry the electronic signals back and forth between the
pulse generator and the heart muscle. Not only do these
electrodes carry the pulses that stimulate the heart muscle to
contract, but they also sense any electrical activity within the
heart and relay that information back to the pulse generator.1
Electric Pulse at the electrode tip, stimulating the heart muscle
[Image courtesy of National Heart Lung and Blood Institute –
National Institute of Health]
One end of the electrode is plugged into the pulse generator. The other end is connected to the
heart muscle using either passive fixation or active fixation.
Active fixation tips (top right) have tiny coils that
“screws” into the heart muscle. Passive fixation tips
(bottom right) look similar to an arrowhead and
contain “barbs” or tines that grab hold of the heart
muscle. No matter what type of tip is used, once a
pacemaker electrode is attached to the heart muscle, it
is seldom removed. After a while, the heart
regenerates tissue around the tip of the electrode,
enclosing the electrode into the heart muscle.
Separating the tip from the heart muscle could be
quite dangerous for the patient. Electrode Tips – Active
fixation (top) and Passive
fixation (bottom)
Southwest Center for Microsystems Education (SCME) Page 10
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
To insert an electrode into the heart, the
surgeon threads the electrodes through a
large vein in the chest, leading into the
heart. For the right atrium or right
ventricle, this vein is normally the
superior vena cava that leads to the right
atrium.
For a single lead pacemaker the
electrode is fed either into the right
atrium or the right ventricle, depending
on the specific heart problem. If the
right atrium needs to be paced, the
electrode tip is placed against the interior
wall of the right atrium. If the right
ventricle needs to be paced, then the
electrode is fed through the tricuspid
valve and the electrode tip is placed in the Electrode placement of two lead pacemaker
lower end of the right ventricle. [Image courtesy of National Heart Lung and Blood
Institute – National Institute of Health]
For a two lead pacemaker, one electrode is connected to the right atrium and the second to the
right ventricle as shown in the graphic above. If a third electrode is needed, this electrode is fed
through a vein that runs around the outside of the heart muscle to the left ventricle where it is
attached. The purpose of this electrode is to synchronize the contractions of the right and left
ventricles and is used for cardiac resynchronization therapy or CRT.
Pacemaker History17,18
As you’ve seen in the previous discussion, there is a lot of
“stuff” inside the pulse generator of a pacemaker and it’s
remarkable that all of that technology has been shrunk to fit
in such a small package. However, that has not always
been the case. The first pacemakers were clunky external
devices. In fact, the first device to be referred to as an
“artificial pacemaker” was developed by American
physiologist Albert Hyman in 1932. This device (shown
right) was powered by a spring-wound hand-cranked motor!
One of the first external pacemakers
©Images in Paediatric Cardiology17
[CC Attribution 3.0]
Southwest Center for Microsystems Education (SCME) Page 11
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
In 1950 a Canadian electrical engineer by the name of John Hopps designed and built an external
pacemaker that used vacuum tube technology. It was “powdered from an AC wall socket and
carried a potential hazard of electrocution of the patient by inducing ventricular fibrillation”.18
During the 1950’s such devices did start getting smaller but it
was the introduction of the silicon transistor that changed the
technology of pacemaker design leading to the first implantable
pacemaker in 1958 developed by surgeon Dr. Ake Senning of the
Karolinska Hospital in Stockholm and physician inventor Rune
Elmqvist.
First Implantable Pacemaker
Profession Marko Turina, University Hospital, Zurich
[CC Attribution 3.0]
In 1960 Birmingham University implanted the first patient controlled variable rate pacemakers
into three patients, all of who “returned to a high quality of life”. By 1966, 56 additional
pacemakers were implanted in to 56 patients with one patient surviving over 5 ½ years.
Progression of Implantable
Pacemakers
[© Images in Paediatric Cardiology
and Medtronics. Used with
permission via Creative Commons
3.0]
Through the years, as microtechnology continued to advance, pacemakers continued to shrink as
you can see in the above image. In 1972 came the development of the hermetically sealed
titanium case as well as the first lithium-iodide cell powered pacemaker (bottom left).
The first rate-responsive pacemakers were designed in the mid-1980’s. “A tiny sensor within the
pacemaker box detected body movement and used this as a surrogate measure of activity. Signals
from the sensor were filtered and applied to an algorithm to alter the pacing rate up or down.
Thus, pacing rate would change according to the patient's activity level.” 17
Southwest Center for Microsystems Education (SCME) Page 12
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
It wasn’t until the 1990’s that we saw the first microprocessor driven pacemakers. These devices
were the first pacemakers with the technology to automatically adjust to the activity level of the
patient by using micro-sized sensors such as the MEMS accelerometers previously discussed.
The 21st century brought about the first bi-ventricular pacing system as well as wireless
monitoring of pacemakers. Pacemaker data can now be downloaded and adjustments made
through a wireless connection at the doctor’s office as well as over a phone line or WiFi.
The Newest in Pacemaker Technology – The Leadless Pacemaker
The first in-human implants of the leadless pacemaker – the Nanostim – were completed in
Prague during December of 2012. A year later (December 2013), another device was approved
and tested by a German group. In February of 2014, St. Jude, in the United States, stated testing
its version of the Nanostim in a clinical trial.20 Clinical trials of the Nanostim in Europe have
cleared the way for this device to be sold commercially; however, it and other leadless
pacemakers are still being investigated in clinical trials in the United States.
The St. Jude Nanostim is less than 10 percent the size of a conventional pacemaker. It is
“approximately 1.5 inches long and under 6 mm in diameter”.21 Since St. Jude’s announcement
in February of 2014, other companies such as Medtronics and Boston Scientific have moved
forward engineering their own leadless devices. By the end of 2014 and into 2015, several
clinical trials were started to test the effectiveness and safety of these devices. Most of these
trails are still on going.
A leadless pacemaker is a small device that is inserted into the heart using a steerable catheter
via the femoral vein. This procedure is less invasive than the procedure to insert a conventional
pacemaker and can be done in about 30 minutes, about one-third of the time it takes to insert a
conventional pacemaker. The absence of leads removes the complications of leads that include
an increase in valve regurgitation due to a heart valve opening being used to insert an electrode
into the ventricle, electrode corrosion, and a faulty electrode.
The self-contained battery of the leadless pacemaker is said to have a nine-year lifespan at 100%
pacing, or more than 13 years at 50% pacing. When the battery runs low, the leadless pacer is
designed to be fully retrievable.22
In August 2015, St. Jude announced the “primary results from the LEADLESS II study that
confirm the positive benefits of the Nanostim leadless pacemaker for patients in need of a single-
chamber ventricular pacemaker”. Out of the 526 participants, 22 experienced adverse devices
effects, all of which were consistent with conventional pacemakers. “After six months, data
from LEADLESS II shows the trial met both endpoints for primary effectiveness and safety. The
study also demonstrated the Nanostim leadless pacemaker's longer-term retrievability and the
device's significant projected battery longevity.”21
Southwest Center for Microsystems Education (SCME) Page 13
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
Summary
As you can see, the microtechnology of pacemakers has provided many advantages for both the
patients and the doctors. The process of implanting a pacemaker is considered out-patient
surgery, and the process of accessing and monitoring pacemaker activity is virtually invisible to
both the patient and the doctor. The patient’s quality of life improves tremendously to the point
where the majority of patients continue living as they did before a pacemaker even became
necessary.
Microtechnology pacemaker sensors and components have become smaller, more reliable and
more sensitive, leading to smaller packages, longer-lasting devices, and improved response to the
physical and emotional demands of the patients. In just the pacemaker alone, you’ve seen
examples of this in the accelerometer sensor, the minute ventilation sensor, the batteries and the
reed switch/sensor.
Glossary
3-axis accelerometer – An accelerometer that measure movement and vibrations along the x, y,
and z axes in a 3-dimensional space.
Accelerometer – An electromechanical device that measures acceleration forces. Such forces
may be static like the continuous force of gravity or dynamic such as movement or vibrations.
Arrhythmia – An irregular heart rhythm or heart rate. Arrhythmia can be a heart rate that is too
fast, too slow, or irregular.
AV Node – The atrioventricular node located in the lower portion of the right atrium of the heart.
The AV node is part of the heart’s electrical signal and is responsible for delaying the signal
from the SA node and sending electrical signals though the ventricles causing them to contract.
Bradycardia – A slower than normal heart rate.
Defibrillator (ICD) – a type of pacemaker used to control abnormal or fast heart rhythms caused
by ventricular tachycardia (a fast heart rate) or ventricular fibrillation.
Electrode – A conductor that allows current to flow toward or away from some part of a circuit.
In the case of a pacemaker, electrodes carry current from the pulse generator to a tip that is
attached to the heart muscle.
Heart block - When the heart’s electrical signals cannot travel through the heart muscle due to
damaged electrical pathways (bundles), blockage within the pathways, or a faulty SA or AV
node.
Minute ventilation - The product of respiratory rate (the number of breaths per minute) and tidal
volume (the lung volume representing the amount of air inhaled and exhaled during normal,
relaxed breathing).
Southwest Center for Microsystems Education (SCME) Page 14
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
Reed Switch – A reed switch consists of two ferromagnetic reeds, hermetically sealed in a dry
inert-gas atmosphere within a glass capsule, thereby protecting the contacts from contamination.
The reeds are sealed in the capsule in cantilever form so that their free ends overlap and are
separated by a small air gap.
Reed Sensor – A reed switch with additional functionally (e.g., ability to withstand higher shock,
easier mounting capability, additional intelligent circuitry)
SA node – The sinus node is located in the upper portion of the right atrium of the heart and is
known as the heart’s natural pacemaker. The SA node initiates the cycle of a heart beat by
sending electronic pulses through the right atrium to the AV node and to the left atrium, causing
both atria to contract.
Transthoracic impedance - The body’s resistance to current flow represented by the skin, fat,
muscle, and lung tissue in a patient’s chest.
References
1.
“Overview of Pacemakers and Implantable Cardioverter Defibrillators (ICDs)”. Johns Hopkins
Medicine. Health Library.
2.
“Pacemakers, Defibrillators and Insertable Cardiac Monitoring Devices”. The University of Chicago
– Medicine. Heart Rhythm Disorders.
3.
“Your Heart and Circulatory System – How They Work”. Mayo Clinic. YouTube. Jun 19, 2013.
4.
“How the heart works”. NHS Choices. YouTube. Nov 16, 2012.
5.
Bradycardia. Mayo Clinic Staff. Mayo Clinic. Diseases and Conditions. May 07, 2014.
6.
Heart Block. Heart Disorders - Arrhythmia. Cleveland Clinic. February 2011.
7.
“Who Needs a Pacemaker?” Explore Pacemakers. National Heart, Lung, and Blood Institute.
February 28, 2012.
8.
“Trends in Cardiac Pacemaker Batteries”. Venkateswara Sarma Mallela, V Ilankumaran, and N.
Srinivasa Rao. Indian Pacing Electrophysiol J. 2004 Oct-Dec; 4(4): 201–212. NCBI/PMC.
9.
Cardiac Pacemaker. Machine-History.com
10.
“Device Offers Hope for Battery-Free Pacemakers”. E. J. Mundell. HealthDay Reporter. U.S. News
Health. Nov 5, 2012.
11.
“Recent Advances of Sensors for Pacemakers”. Wei Vivien Shi. MengChu Zhou. ECE Dept. New
Jersey Institute of Technology. 2011 International Conference of Networking, Sensing and Control.
April 11-13, 2011.
12.
“Rate responsive pacing using a minute ventilation sensor.” Mond H., Strathmore N., Kertes P, Hunt
D, Baker G. Pacing Clin Electrophysiol. 1988 Nov;11(11 Pt 2):1866-74. NCBI / PubMed.
13.
“Thoracic Impedance Measurements Can Interfere with Impedance-Based Rate-Responsive
Pacemakers.” Health Devices Sep-Oct 1997;26(9-10):393-4.
14.
“Rate-modulated cardiac pacing based on transthoracic impedance measurements of minute
ventilation: Correlation with exercise gas exchange.” G. Neal Kay MD, Rosemary S. Bubien, RN,
MSN, Andrew E. Epstein, MD, FACC, Vance J. Plumb, MD, FACC. Journal of the American
College of Cardiology. Volume 14, Issue 5, 1 November 1989, Pages 1283–1289.
15.
“Teardown of the Apply iPhone 4 Smart Phone”. Chipworks. Chipworks, Inc. June 23, 2010.
16.
“MD&M West 2015: Coto Releases RedRock RR100 MEMS-Based Magnetic Reed Sensor”. Azo
Nano. MEMS-NEWS. February 11, 2015. (Original source: Coto Technology.
17.
“A Brief History of Cardiac Pacing”. Images Paediatr Cardiol. 2006 Apr-Jun; 8(2):17-81.
18.
“Artificial Cardiac Pacemaker”. Wikipedia. March 16 2015.
Southwest Center for Microsystems Education (SCME) Page 15
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
19.
Conversation with Todd Christenson. President and CTO. HT MicroAnalytical, Inc. Albuquerque,
NM. February 2015.
20.
“Capsule-Sized Leadless Pacemakers May Lead the Way in Heart Rhythm Management.” Burt
Cohen. AngioPlasty.org. February 6, 2014.
21.
“St. Jude Medical Announces Primary Results from LEADLESS II study.” RTT News. August 30,
2015. Nasdaq.
22.
“St. Jude Medical Continues Leadless Pacemaker Push”. Stephen Levy. March 19, 2014.
Cardiovascular. Qmed.
The information contained herein is considered to be true and accurate; however the Southwest Center for
Microsystems Education (SCME) makes no guarantees concerning the authenticity of any statement. SCME accepts
no liability for the content of this unit, or for the consequences of any actions taken on the basis of the information
provided.
Support for this work was provided by the National Science Foundation’s Advanced Technological Education
(ATE) program. For more learning modules related to microtechnology, visit the SCME website (http://scme-
nm.org).
Southwest Center for Microsystems Education (SCME) Page 16
Pacemakers_PK2_Jan2018 The Microtechnology of Pacemakers PK
You might also like
- Shrapnel White PaperDocument42 pagesShrapnel White PaperdelgiudiceinvestimentiNo ratings yet
- BaddiDocument43 pagesBaddi01copy100% (3)
- ISO 9001-2015 Mandatory Documents RequiredDocument1 pageISO 9001-2015 Mandatory Documents RequiredAnonymous GWDhNTOKO100% (5)
- Ultima Underworld 2 - Player's GuideDocument40 pagesUltima Underworld 2 - Player's GuideThea_VatarNo ratings yet
- ELCOMA Diractory 2022 2024 1Document96 pagesELCOMA Diractory 2022 2024 1Unmesh ThoratNo ratings yet
- 2 Maps TypesDocument22 pages2 Maps TypesSiphumeze TitiNo ratings yet
- ++ 437-1692-1-PBDocument9 pages++ 437-1692-1-PBAli TarkashvandNo ratings yet
- Design and Analysis of A Dual Chamber Cardiac Pacemaker Using VHDL in Biomedical ApplicationDocument3 pagesDesign and Analysis of A Dual Chamber Cardiac Pacemaker Using VHDL in Biomedical ApplicationEditor IJRITCCNo ratings yet
- Cardiac PacemakersDocument3 pagesCardiac PacemakersDhruv SridharNo ratings yet
- SBM1403 Unit1Document64 pagesSBM1403 Unit1Balasaraswathi SNo ratings yet
- Q: Characterize The Different Types of Pacemaker. Explain The Various Steps of PacingDocument4 pagesQ: Characterize The Different Types of Pacemaker. Explain The Various Steps of PacingKRUPALITHAKKAR100% (1)
- Mount Zoin Engineering & Technology Cycle Test Ii Medical Electronic-Ec1006Document11 pagesMount Zoin Engineering & Technology Cycle Test Ii Medical Electronic-Ec1006bhagyampNo ratings yet
- Artificial PacemakerDocument17 pagesArtificial PacemakerNEX456No ratings yet
- Microcontroller Based Heart Rate Meter: Bachelor of Technology IN Electronics and Communication EngineeringDocument41 pagesMicrocontroller Based Heart Rate Meter: Bachelor of Technology IN Electronics and Communication EngineeringKrupal Kumar100% (1)
- IEEEDocument5 pagesIEEESanjana GururajNo ratings yet
- Converts: That Energy From One Form To AnotherDocument2 pagesConverts: That Energy From One Form To AnotherSalim EchbarbiNo ratings yet
- Recent Trends in Medical Imaging by Using VLSIDocument4 pagesRecent Trends in Medical Imaging by Using VLSIinventyNo ratings yet
- Report On Hospital TrainingDocument35 pagesReport On Hospital TrainingChitralekha GaneshNo ratings yet
- MEMS Notes..Document10 pagesMEMS Notes..abhi shekNo ratings yet
- Basics of ElectrocardiographyDocument10 pagesBasics of ElectrocardiographyThanh Nhan LeNo ratings yet
- Pacemaker BatteriesDocument12 pagesPacemaker Batteriesrohit860No ratings yet
- Prepared By: Kiran WaliaDocument28 pagesPrepared By: Kiran WaliaAdityaNo ratings yet
- 31STDocument12 pages31STGSMPNo ratings yet
- 05 - Smart Epilepsy Prediction SystemDocument9 pages05 - Smart Epilepsy Prediction SystemAkhil GanganNo ratings yet
- PacemakerDocument12 pagesPacemakerkumargpalaniNo ratings yet
- Embedded Systems in Medical Applications: Arpitha Mukundraj:4MC20EE004 Nirikshitha S:4MC20EE051 Priyanka K P:4MC20EE062Document12 pagesEmbedded Systems in Medical Applications: Arpitha Mukundraj:4MC20EE004 Nirikshitha S:4MC20EE051 Priyanka K P:4MC20EE062Rakshith HsNo ratings yet
- Semester Project ReportDocument11 pagesSemester Project ReportAfaque Ali (F-Name Rashid Ali)No ratings yet
- What Is A Pacemaker?Document4 pagesWhat Is A Pacemaker?Abhishek MishraNo ratings yet
- Primus DescriptionDocument92 pagesPrimus Descriptionwizardgrt1No ratings yet
- Pacemaker: Presented byDocument16 pagesPacemaker: Presented bynivethitha priyadharshiniNo ratings yet
- Smart Bio-Medical Signal Monitoring SystemDocument4 pagesSmart Bio-Medical Signal Monitoring SystemIJSTENo ratings yet
- Wijaya 2020 IOP Conf. Ser. Mater. Sci. Eng. 835 012053Document13 pagesWijaya 2020 IOP Conf. Ser. Mater. Sci. Eng. 835 012053Hasnaoui AsmaaNo ratings yet
- 1) Discuss The Working Principle of A MEMS Sensor For Object DetectionDocument6 pages1) Discuss The Working Principle of A MEMS Sensor For Object DetectionvenkyNo ratings yet
- Zurbuchen 2016Document6 pagesZurbuchen 2016Martha H.TNo ratings yet
- PACEMAKERDocument5 pagesPACEMAKERPoonam ThakurNo ratings yet
- Understanding The Heart's Electrical System: Cardiac PacemakerDocument5 pagesUnderstanding The Heart's Electrical System: Cardiac PacemakerDitaMaryaniNo ratings yet
- Pacemaker NotesDocument7 pagesPacemaker Notesrohit860No ratings yet
- Olarewaju, Victor - BME 503 ASS 1Document6 pagesOlarewaju, Victor - BME 503 ASS 1olawajuvictorNo ratings yet
- Electronic Devices For MEDocument19 pagesElectronic Devices For METallha AhmedNo ratings yet
- Micro ReportDocument8 pagesMicro ReportWasan ShakirNo ratings yet
- Bio Intrumentation Lab 01Document10 pagesBio Intrumentation Lab 01roroy43581No ratings yet
- BRAIN PORT (A Neural Network Visual Device) 1Document30 pagesBRAIN PORT (A Neural Network Visual Device) 1Priya Mandal0% (1)
- Sensors Used in Medical FieldDocument15 pagesSensors Used in Medical FieldVidhya Shankar100% (1)
- Medical Instrumentation II - Defib and PacemakerDocument14 pagesMedical Instrumentation II - Defib and PacemakerRula BastoniNo ratings yet
- Jitesh Interview ScriptDocument14 pagesJitesh Interview ScriptHRISHIKESH DHANDENo ratings yet
- Human Heart Modelling Using Hydro Electro Mechanical SystemsDocument25 pagesHuman Heart Modelling Using Hydro Electro Mechanical Systemsmaggi3012No ratings yet
- MechatronicsDocument26 pagesMechatronicsGlen Peter Miranda100% (2)
- ECG CardiotachometerDocument43 pagesECG CardiotachometerWillius David DjoNo ratings yet
- MC LAB MiniProjectDocument2 pagesMC LAB MiniProjectGracefully BluntNo ratings yet
- Health Parameters Monitoring SystemDocument4 pagesHealth Parameters Monitoring SystemEditor IJRITCCNo ratings yet
- Lingaya'S Institute of Management and TechnologyDocument25 pagesLingaya'S Institute of Management and Technologysonaldeep singhNo ratings yet
- ECG Analysis SystemDocument16 pagesECG Analysis SystemSurabhi MishraNo ratings yet
- Pacemaker InformatiiDocument4 pagesPacemaker InformatiiAndreea MitranNo ratings yet
- TestsDocument8 pagesTestsNamrathaThalatoti ywSSAmHsULNo ratings yet
- Pressure Sensing TransmitterDocument10 pagesPressure Sensing TransmitterKrishnamurthy AnantharamakrishnanNo ratings yet
- 19 Vol 92 No 1Document9 pages19 Vol 92 No 1lahlouhNo ratings yet
- Gaetano L'Episcopo: Introduction To MEMS and NEMSDocument54 pagesGaetano L'Episcopo: Introduction To MEMS and NEMSGaetanoNo ratings yet
- About Mems:: What Is MEMS Technology?Document14 pagesAbout Mems:: What Is MEMS Technology?ashabiaNo ratings yet
- A Project Report ON Ecg Signal Denoisig Using Wavelet TransformDocument58 pagesA Project Report ON Ecg Signal Denoisig Using Wavelet Transformnaali pavanNo ratings yet
- 00 Micromechatronic SystemsDocument47 pages00 Micromechatronic SystemscacaNo ratings yet
- Ei 65 Unit 5Document31 pagesEi 65 Unit 5karthiha12No ratings yet
- 6 Engine Computer Systems2Document36 pages6 Engine Computer Systems2Khairulz AnuarNo ratings yet
- Medicaps University Session - 2019: Digital Signal ProcessingDocument9 pagesMedicaps University Session - 2019: Digital Signal ProcessingPrerit KavthekarNo ratings yet
- Power Electronics Applied to Industrial Systems and Transports: Volume 5: Measurement Circuits, Safeguards and Energy StorageFrom EverandPower Electronics Applied to Industrial Systems and Transports: Volume 5: Measurement Circuits, Safeguards and Energy StorageNo ratings yet
- EKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXFrom EverandEKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXRating: 5 out of 5 stars5/5 (2)
- Artificial Intelligence for Computational Modeling of the HeartFrom EverandArtificial Intelligence for Computational Modeling of the HeartTommaso MansiNo ratings yet
- ĐỀ SỐ 9 đên 16Document28 pagesĐỀ SỐ 9 đên 16Phương Thảo HàNo ratings yet
- PM CLINIC WB97R-5E0 SN F90001-Up 05102017Document3 pagesPM CLINIC WB97R-5E0 SN F90001-Up 05102017efrain.hilario.86No ratings yet
- Group-1 1BM19EC020 044 045 052Document15 pagesGroup-1 1BM19EC020 044 045 052Vineet ANo ratings yet
- Print 3Document2 pagesPrint 3Li ReNo ratings yet
- Gc2300 SeriesDocument19 pagesGc2300 SeriesRyan DosseyNo ratings yet
- Linear Programming (LPP) Transporatation Problem: Step 1: Formulate The ProblemDocument2 pagesLinear Programming (LPP) Transporatation Problem: Step 1: Formulate The ProblemAnik GuinNo ratings yet
- Green ComputingDocument7 pagesGreen Computingerwin.dee.cicsNo ratings yet
- Access Network Reveolution XDSL Principles and ApplicationsDocument57 pagesAccess Network Reveolution XDSL Principles and ApplicationsMohamed ShabanaNo ratings yet
- 2000 High Speed CMOS Data DL129-D c20000324Document408 pages2000 High Speed CMOS Data DL129-D c20000324Dara Nyara Ricardo SocorroNo ratings yet
- Risk Assesment - MobilisationDocument7 pagesRisk Assesment - Mobilisationشاز إياسNo ratings yet
- Databricks Data Processing Addendum 25 Sept 2021 FINALDocument12 pagesDatabricks Data Processing Addendum 25 Sept 2021 FINALVaibhav AntilNo ratings yet
- Method and Means For Adjusting The Elevation of Manhole Covers - MCCOY ARCHIBADocument5 pagesMethod and Means For Adjusting The Elevation of Manhole Covers - MCCOY ARCHIBALandel SmithNo ratings yet
- Assignment 4 Assn4 PDFDocument4 pagesAssignment 4 Assn4 PDFcybernerdNo ratings yet
- Kerberos FunctionalityDocument8 pagesKerberos Functionalityjimm gamerNo ratings yet
- CSC340 CN CDF V3.1Document2 pagesCSC340 CN CDF V3.1Faizan KhanNo ratings yet
- Tnteu-Icssr Paper List 803Document803 pagesTnteu-Icssr Paper List 803shahidafzalsyedNo ratings yet
- Oel Lab 1Document4 pagesOel Lab 1dzikrydsNo ratings yet
- 3 ERP Software RFP Exhibit GDocument575 pages3 ERP Software RFP Exhibit GJacob YeboaNo ratings yet
- 21 Architecture PDFDocument19 pages21 Architecture PDFjayaprasanna123No ratings yet
- Big Bang Theory - Pass The ButterDocument6 pagesBig Bang Theory - Pass The ButterAlan PereiraNo ratings yet
- TVL ICT ANIMATION-NCII Q1 MODULE-2 PassedDocument11 pagesTVL ICT ANIMATION-NCII Q1 MODULE-2 PassedBook_AinjelleNo ratings yet
- Local Balloon Professional Earns Recognition As Certified Balloon ArtistDocument2 pagesLocal Balloon Professional Earns Recognition As Certified Balloon ArtistPR.comNo ratings yet
- Blockchain Preparation Audit ProgramDocument7 pagesBlockchain Preparation Audit ProgramVigorNo ratings yet
- Lead Scoring Case Study PresentationDocument11 pagesLead Scoring Case Study PresentationDevanshi100% (2)