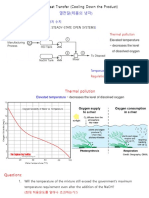
Professional Documents
Culture Documents
BalanceEqnsAndMatModels in
BalanceEqnsAndMatModels in
Uploaded by
tienkhoaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BalanceEqnsAndMatModels in
BalanceEqnsAndMatModels in
Uploaded by
tienkhoaCopyright:
Available Formats
Generalized Balance Equations - thermofluids.
net
Phuong trinh can bang tong quat
Mass: Khoi luong
dm kg
dt
m i
i me
e s ; (1)
Rate of increase of mass Mass flow rate Mass flow rate
in an open system. into the system. out of the system.
Ty le tang khoi luong Toc do dong chay Toc do dong chay khoi
trong 1 he thong mo
AV vao he thong
kg luong ra khoi he thong
where, m AV K
v
Energy: Nang luong
Nang luong duoc van chuyen
boi dong chay khoi luong ra ngoai
dE
m j i i m j
e e Q Wext kW (2)
dt i e Rate of heat Rate of external
Rate of increase Energy transported Energy transported transfer into work transfer
of stored energy by mass flow in. by mass flow out. Toc do truyen nhiet the system. out of the system.
of the open system. Ty le chuyen cong viec
Ty le tang nang luong Nang luong duoc van chuyen vao he thong
ben ngoai ra khoi he thong
gz kJ
du tru cua he thong mo boi dong chay khoi luong trong 2
V
where, j h ke pe h
2000 1000 kg
N VI
and Wext Wsh Wel WB ; Wsh 2 T ; Wel kW
60 1000
W
WB pdV kJ ; WB lim B ; kW
t 0 t
Entropy:
Nang luong duoc van chuyen
boi dong chay khoi luong ra ngoai
dS Q kW
dt
m s
i
i i m s
e
e e
TB
Sgen K
Rate of
Rate of increase Entropy Entropy Entropy generation of
of entropy for transported by transported by transferred entropy inside
an open system. mass flow in. mass flow out. by heat. the system (3)
Ty le tang entropy Nang luong duoc van chuyen Nang luong boundary.
doi vs 1 he thong mo boi dong chay khoi luong trong truyen nhiet ty le tao ra entropy ben
where, according to the second law, Sgen 0 trong ranh gioi he thong
theo dinh luat thu hai
Phuong trinh so du tuy chinh
Customized Balance Equations - thermofluids.net
Closed Steady Systems (Wall, Light bulb, Laptop adapter, Gear box, closed cycles)
He thong on dinh khep kin (Tuong, Bong den, Bo dieu hop may tinh xach tay, Hop so, chu trinh khep kin)
Mass Equation: m constant (1)
Energy Equation: 0 Q Wext kW where, Wext WB Wsh Wel (2)
Q kW
Entropy Equation: 0 Sgen ; Second law asserts: Sgen 0 (3)
TB K Dinh luat thu 2 khang dinh
Single-Flow Open-Steady Systems (pumps, turbines, nozzles, valves, pipes, etc.)
He thong on dinh mo 1 dong chay (may bom, tuabin, voi phun, van, duong ong, v.v.)
kg
Mass: me mi m s (1)
Energy: mje mji Q Wext kW
V2 gz (2)
where, j h ke pe h kJ/kg , Wext WB Wsh Wel kW
2000 1000
Q kW
Entropy: mse msi Sgen where, by second law, Sgen 0 (3)
TB K
Closed Processes (Heating water in a tank, piston-cylinder compression)
Quy trinh khep kin (Lam nong nuoc trong bon chua, nen piston-xi lanh)
Mass: m constant kg (1)
Energy: E E f Eb Q Wext or, me Q Wext kJ
V2 gz (2)
where, e u ke pe u kJ/kg ; Wext WB Wsh Wel kJ
2000 1000
Entropy: S S f Sb Q / TB Sgen or, ms Q / TB Sgen kJ/K where, Sgen 0 (3)
Open Processes (Filling an evacuated tank, filling a propane cylinder, discharge from a tank)
Quy trinh mo (Do day be chua da hut chan khong, lam day xi lanh propan, xa tu be chua)
Mass: m m f mb mi me kg (1)
Energy: E E f Eb mi ji me je Q Wext kJ
V2 gz V2 gz
where, e u ke pe u , j h ke pe h kJ/kg (2)
2000 1000 2000 1000
and Wext WB Wsh Wel kJ
Entropy: S S f Sb mi si me se Q / TB Sgen where, Sgen 0 (3)
Danh gia trang thai thu cong
Manual State Evaluation
thermofluids.net>Tables
Cong thuc lien quan den trang thai chung: (ap dung cho bat ky chat nao)
General State Related Equations: ( applies to any substance)
1 V2 gz
m V ; ; ke ; pe ; e u ke pe ; j h ke pe ; h u pv (1)
v 2000 1000
E me ;; S ms ; KE m ke ; PE m pe (2)
m e ; S m
AV ; V AV ; E m s; (3)
Tds du pdv dh vdp ; cv u / T v ; c p h / T p (4)
SL Model: (Assumptions: constant cv =constant: see Tables>Table-A)
u u2 u1 c(T2 T1 ) ; cv c p c; (5)
h h2 h1 (u pv) u (pv) c(T2 T1 ) v(p2 p1 ) (6)
T
s c p ln 2 (7)
T1
PG Model: (Assumptions: p RT ; cv =constant: see Tables>Table-C)
RT m m R m T T R
p RT RT T R nR , where R (8)
v V V M M V V M
u u2 u1 cv (T2 T1 ) , h h2 h1 c p(T2 T1) , where cp (cv R) (9)
T p T v c kR R
s c p ln 2 R ln 2 ; s cv ln 2 R ln 2 ; also, k p , c p ; and cv (10)
T1 p1 T1 v1 cv k 1 k 1
k k 1
k k k k 1 k
p T k 1 v V T p k v p v
s con process: 2 2 2 1 1 ; 2 2 1 ; 2 1 ; (11)
p1 T1 1 v2 V2 T1 p1 v2 p1 v2
For polytropic process replace k with n Doi vs qua trinh thay the da huong k vs n
IG Model: (Assumptions: p RT ; cv is function of T : see Tables>Table-D)
RT m m R m T T
p RT RT T R nR (12)
v V V M M V V
h h T , u u T s s p, T (use ideal gas tables); c p cv R (13)
Phan phu thuoc nhiet do cua entropi duoc tach ra khoi phan phu thuoc ap suat:
The temperature dependent part of entropy is separated from the pressure dependent part:
T2
dT p p
s cp R ln 2 s (T2) s (T1) R ln 2 , where s (T ) is tabulated against T . (14)
o o 0
T1
T p1 p1
PC Model: (see Tables>Table-B) Determine the phase, L, V or M, of the fluid. For vapor use superheated Table.
For mixture, use saturation table (if the quality is not known, your goal should be to evaluate the quality
first which is the key to finding all specific properties of a mixture). For liquid use the CL sub-model.
CL Sub-Model: v , u and s depend on T only. Therefore, use the temperature-sorted saturation table to
obtain v v f @T , u u f @T or s s f @T . To find h , use h u pv u f @T pv f @T .
RG Model: (see Tables>Table-E) p Z ( pr , Tr ) RT where Z, the compressibility factor, is obtained from a
chart. pr and Tr are pressure and temperature normalized by the corresponding critical properties. Just
like entropy in the PG or IG model, h and u also have two parts, one temperature dependent and another
pressure dependent, in the RG model. The departure of these values from the corresponding IG values are
tabulated in the enthalpy and entropy departure charts as functions of pr and Tr . Therefore, the complete
state can be evaluated if pr and Tr are given.
You might also like
- Crude CharacterizationDocument76 pagesCrude Characterizationavciay100% (1)
- Documents - Tips - Final Draft Thermo000 PDFDocument222 pagesDocuments - Tips - Final Draft Thermo000 PDFYoshua Martel Candava77% (13)
- Energy Balance SKDocument38 pagesEnergy Balance SKMegaraj ReddyNo ratings yet
- Strength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresFrom EverandStrength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresRating: 4 out of 5 stars4/5 (1)
- Elementary Reactor Physics: The Commonwealth and International Library: Nuclear Engineering DivisionFrom EverandElementary Reactor Physics: The Commonwealth and International Library: Nuclear Engineering DivisionNo ratings yet
- Multicomponent DistillationDocument11 pagesMulticomponent DistillationManuel Rodriguez ValenciaNo ratings yet
- Umesh Rajoria's Notes Class 11 CombinedDocument324 pagesUmesh Rajoria's Notes Class 11 Combinedpillisathwik2100% (3)
- IES Previous Year Thermodynamics SolutionDocument228 pagesIES Previous Year Thermodynamics SolutionchandankrdumkaNo ratings yet
- Notes Gas AbsorptionDocument15 pagesNotes Gas AbsorptionPeter Paul BucsitNo ratings yet
- Natural Gas PresentationDocument30 pagesNatural Gas PresentationEng Said ElsayedNo ratings yet
- Entropy Balance: Prof. Dr. Uğur AtikolDocument14 pagesEntropy Balance: Prof. Dr. Uğur AtikolRajesh ShuklaNo ratings yet
- Paramagnetism Spin One HalfDocument42 pagesParamagnetism Spin One Halfpesta0% (1)
- Chapter 1 - Part 2 - 2Document10 pagesChapter 1 - Part 2 - 2حسن كميت hassankomeit lNo ratings yet
- CH 4 Energy Transport by Heat Work MassDocument47 pagesCH 4 Energy Transport by Heat Work MassgfsfNo ratings yet
- Thermodynamics ReviewDocument47 pagesThermodynamics ReviewZain AhmedNo ratings yet
- Week/day 8: Entropy BalanceDocument31 pagesWeek/day 8: Entropy Balanceronni bermudezNo ratings yet
- Energy Balance 2Document42 pagesEnergy Balance 2mymamforeverNo ratings yet
- Topic 1 - Intro Heat Transfer - RevDocument46 pagesTopic 1 - Intro Heat Transfer - RevNUR ADILAH BINTI MOHAMADNo ratings yet
- 화학공학입문설계 강의노트 9Document19 pages화학공학입문설계 강의노트 9wani anaNo ratings yet
- Mass and Energy Analysis of Control VolumesDocument22 pagesMass and Energy Analysis of Control VolumesYeiLan StasiaNo ratings yet
- Chapter 7.5 - EntropyDocument11 pagesChapter 7.5 - EntropyhudarusliNo ratings yet
- EntropyDocument30 pagesEntropyفضائح لا تصدقNo ratings yet
- CH3 - 1st Law of Thermodynamics Closed SystemDocument61 pagesCH3 - 1st Law of Thermodynamics Closed SystemDeacon ChiaNo ratings yet
- Chapter 4 - First Law of ThermodynamicsDocument29 pagesChapter 4 - First Law of ThermodynamicsUSAIMAH SHARIFNo ratings yet
- Chapter 2-2Document20 pagesChapter 2-2Najmul Puda PappadamNo ratings yet
- Molecular Spectroscopy: Office: EmailDocument79 pagesMolecular Spectroscopy: Office: EmailLeeNo ratings yet
- 3 Mass Balance Agro1 PDFDocument28 pages3 Mass Balance Agro1 PDFLiam LagartoNo ratings yet
- Chapter 7 EntropyDocument41 pagesChapter 7 Entropyrustam effendyNo ratings yet
- Entropy: T DS QDocument12 pagesEntropy: T DS QUsama Jahangir KhanNo ratings yet
- MFGE 4315 5315 Lecture 3Document12 pagesMFGE 4315 5315 Lecture 3TAWHIDNo ratings yet
- Classical and Quantum Statistics: MB, BE & FD Statistics: Dr. Neelabh SrivastavaDocument22 pagesClassical and Quantum Statistics: MB, BE & FD Statistics: Dr. Neelabh Srivastavasheepriyanka322No ratings yet
- Flow of Fluid and Bernoulli's EquationDocument27 pagesFlow of Fluid and Bernoulli's EquationZac IriberriNo ratings yet
- Entropy View of Real Engineering Process .Document23 pagesEntropy View of Real Engineering Process .Muket AgmasNo ratings yet
- Statistical MechanicsDocument41 pagesStatistical MechanicsFrazNo ratings yet
- StatisticsDocument34 pagesStatisticsPoth BaliyanNo ratings yet
- ThermodynamicsDocument67 pagesThermodynamicsHimanshu RaiNo ratings yet
- Introduction Molecular Spectroscopy BSc-Lect - 1Document30 pagesIntroduction Molecular Spectroscopy BSc-Lect - 1Almas FatimaNo ratings yet
- Part 3B - Energy Balance - Open SystemDocument23 pagesPart 3B - Energy Balance - Open SystemHarold SumagaysayNo ratings yet
- Basic ThermoDocument22 pagesBasic ThermoRoselyn BoNo ratings yet
- Dzexams 2as Physique As - t1 20191 1508735Document5 pagesDzexams 2as Physique As - t1 20191 1508735Qlash Mouad CrNo ratings yet
- Applications of The First Order Differential EquationsDocument6 pagesApplications of The First Order Differential EquationsJake BarettoNo ratings yet
- Unit-I Basic Concepts and First Law: ThermodynamicsDocument42 pagesUnit-I Basic Concepts and First Law: ThermodynamicsShivam Kumar Singh 18BME0066No ratings yet
- The First Law of Thermodynamics - 040170170000Document50 pagesThe First Law of Thermodynamics - 040170170000xixoNo ratings yet
- Unit 4 - Topic 5Document38 pagesUnit 4 - Topic 5sgmdhussainNo ratings yet
- Module 1 - Measurement in PhysicsDocument6 pagesModule 1 - Measurement in PhysicsTOP ERNo ratings yet
- Squid Lab Packet v3Document8 pagesSquid Lab Packet v3Bradley Chung100% (1)
- EntropyDocument30 pagesEntropyteddiyfentawNo ratings yet
- Reminder: The General Balance Equation: Accumulation Creation - Destruction + Flow in - Flow OutDocument14 pagesReminder: The General Balance Equation: Accumulation Creation - Destruction + Flow in - Flow OutforemarNo ratings yet
- Quantum Coherence in Photo-Ionization With Tailored XUV PulsesDocument11 pagesQuantum Coherence in Photo-Ionization With Tailored XUV PulsesraveneyesdeadNo ratings yet
- Unit II - 2Document89 pagesUnit II - 2physicist sharmaNo ratings yet
- Ch-3 Unit & MeasurementDocument22 pagesCh-3 Unit & MeasurementRakesh KumarNo ratings yet
- Research On Control Volume Analysis That Are Required To Select Any Engineering Applications (Turbines, Compressors, Pumps, Nozzles, DiffusersDocument11 pagesResearch On Control Volume Analysis That Are Required To Select Any Engineering Applications (Turbines, Compressors, Pumps, Nozzles, DiffuserszohairahmedNo ratings yet
- mcl721 21Document25 pagesmcl721 21Vivek MahindrakarNo ratings yet
- Newton's Second Law: F Ma V MV F Ma M T TDocument75 pagesNewton's Second Law: F Ma V MV F Ma M T TNA TbNo ratings yet
- Current Electricity: Electrical Conductors and InsulatorsDocument14 pagesCurrent Electricity: Electrical Conductors and InsulatorsPhilip MooreNo ratings yet
- 冷凍工程及實習 Experiments in Refrigeration EngineeringDocument65 pages冷凍工程及實習 Experiments in Refrigeration EngineeringMuhammad RaflyNo ratings yet
- Quantum Computing: The Future of Information Processing: The Science CollectionFrom EverandQuantum Computing: The Future of Information Processing: The Science CollectionNo ratings yet
- Energy Chronicles: Keys to understanding the importance of energyFrom EverandEnergy Chronicles: Keys to understanding the importance of energyNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 6: Gravitational and Inertial Control, #6From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 6: Gravitational and Inertial Control, #6No ratings yet
- Liquid Glass Transition: A Unified Theory From the Two Band ModelFrom EverandLiquid Glass Transition: A Unified Theory From the Two Band ModelNo ratings yet
- Lecture 15Document33 pagesLecture 15Anas Nasir officalNo ratings yet
- InTech-Mass and Heat Transfer During Thin Film Evaporation of Liquid SolutionsDocument17 pagesInTech-Mass and Heat Transfer During Thin Film Evaporation of Liquid SolutionsClarence AG YueNo ratings yet
- Fundamentals of Reservoir Fluids: Norman Clark ClarkDocument35 pagesFundamentals of Reservoir Fluids: Norman Clark ClarkFarid AndriadiNo ratings yet
- Compilation of ProblemsDocument14 pagesCompilation of ProblemsYnnoNo ratings yet
- User GuideDocument191 pagesUser GuideDiego CoronaNo ratings yet
- 5 Sem Booklet 2020 PDFDocument31 pages5 Sem Booklet 2020 PDFAryan PatelNo ratings yet
- Experiment 4 Binary Phase Diagrams 2022Document5 pagesExperiment 4 Binary Phase Diagrams 2022Rey DLRNo ratings yet
- Chemical Engineering & Processing: Process Intensi Fication: ArticleinfoDocument9 pagesChemical Engineering & Processing: Process Intensi Fication: Articleinfomiza adlinNo ratings yet
- Group A5 - EXP 5 Batch Packed DistillationDocument35 pagesGroup A5 - EXP 5 Batch Packed DistillationKabilashini Mana Mohan100% (3)
- FugsDocument60 pagesFugsFaisal MumtazNo ratings yet
- Capítulo 2, TermodinámicaDocument64 pagesCapítulo 2, TermodinámicaISABELLA CUESTAS ACOSTANo ratings yet
- Lect 1 & 2introduction To ThermodynamicsDocument25 pagesLect 1 & 2introduction To ThermodynamicsMupenziNo ratings yet
- S Announcement 6387Document1 pageS Announcement 6387Shalisa La Raine RoxasNo ratings yet
- Development of A Low-Friction Radial Shaft Seal UsDocument15 pagesDevelopment of A Low-Friction Radial Shaft Seal UsAnissa LamraniNo ratings yet
- Chapter 07 - Trace ElementsDocument41 pagesChapter 07 - Trace ElementsJean D. MARIN PADILLLANo ratings yet
- Fundamental Modeling and Simulation of A Binary Continuous Distillation ColumnDocument6 pagesFundamental Modeling and Simulation of A Binary Continuous Distillation ColumnRohitThakranNo ratings yet
- Module 2. Solutions Thermodynamics - Part 3Document46 pagesModule 2. Solutions Thermodynamics - Part 3VanNo ratings yet
- tối ưu tách nước bằng TEGDocument10 pagestối ưu tách nước bằng TEGTu Dang TrongNo ratings yet
- Select Thermodynamic Models For Process Simulation - A Practical Guide To A Three Steps MethodologyDocument12 pagesSelect Thermodynamic Models For Process Simulation - A Practical Guide To A Three Steps MethodologyBegenkz100% (1)
- SPE 77502 Application of Transient Multiphase Compositional Tracking For Pipeline Flow AnalysisDocument10 pagesSPE 77502 Application of Transient Multiphase Compositional Tracking For Pipeline Flow AnalysisAyauwu LovedayNo ratings yet
- ECUST PROII Advanced Training PDFDocument118 pagesECUST PROII Advanced Training PDFframon.chem35No ratings yet
- Single Stage DistillationDocument15 pagesSingle Stage DistillationFatimah MauludiyahNo ratings yet
- B.Tech 2nd Yr CHDocument22 pagesB.Tech 2nd Yr CHRobinNo ratings yet
- PAPER Final FractionationDocument7 pagesPAPER Final FractionationkaruniaNo ratings yet
- Research of VLE PDFDocument131 pagesResearch of VLE PDFRegiyanti RNo ratings yet
- Enthalpy of Dissociation and Hydration Number of Methane Hydrate From The Clapeyron EquationDocument9 pagesEnthalpy of Dissociation and Hydration Number of Methane Hydrate From The Clapeyron Equationomeo habibNo ratings yet