Professional Documents
Culture Documents
Thermodynamics Formula Sheet
Uploaded by
donny wuOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamics Formula Sheet
Uploaded by
donny wuCopyright:
Available Formats
Thermodynamics - Formula Sheet
1 atm = 101.325 kPa 0°C = 273.15 K
First Law
Ideal Gas Law: pV nRT
First Law: U w q
Equipartition Theorem: Each quadratic contribution to energy gives rise to an average energy of ½kBT
Work:
Isothermal Reversible Process: ln
Enthalpy: H U PV
Heat Capacities: dU cV dT dH cP dT , ,
cp
Adiabatic Process: and
cv
1
Adiabatic Process (cont.): TV constant, pV = constant, and p1 T = constant
Second Law
Second Law: SUniverse S SSur 0
qrev ∆
Entropy Formulations: S ∆ S kB ln W
T
Entropy Changes: ∆ , ln (or ,
V2 P2
Isothermal Entropy Change: S nR ln nR ln
V1 P1
Free Energy
Free Energy: A U TS G H TS dG dH TdS
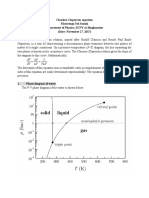
Phase Transitions
dp trsS
Clapeyron Equation:
dT trsV
fus H T fus H
Solid-Liquid Phase Boundary: p p* ln but T T* p p* (T T * )
fus V T* T *
fus V
H 1 1
Liquid-Vapour or Solid-Vapour: p p *e where trs
R T T*
2017 Chemistry Final Selection Examination P ag e 9 of 22
Australian Science Innovations ABN 81731558309
You might also like
- Chapter 04 Physical TransformationsDocument40 pagesChapter 04 Physical TransformationsRuby DalyNo ratings yet
- Kesetimbangan Fasa Dan Diagram FasaDocument13 pagesKesetimbangan Fasa Dan Diagram FasaNidia NidiaNo ratings yet
- PVT Behavior of Fluida & EOSDocument53 pagesPVT Behavior of Fluida & EOSEka WahyuNo ratings yet
- Chapter8 Real Gases and Mixture of GasesDocument26 pagesChapter8 Real Gases and Mixture of GasesMUHAMMED FAISALNo ratings yet
- Carnot Cycle Pressure-Volume DiagramsDocument13 pagesCarnot Cycle Pressure-Volume DiagramsVijeethira RavichandranNo ratings yet
- Lecture 2 EDocument8 pagesLecture 2 EMihai MirceaNo ratings yet
- Entropy View of Theoretical Processes .Document11 pagesEntropy View of Theoretical Processes .Muket AgmasNo ratings yet
- Phase boundaries and vapor pressureDocument7 pagesPhase boundaries and vapor pressureRa saNo ratings yet
- PVT Behavior of Pure FluidsDocument8 pagesPVT Behavior of Pure FluidsNikko ManaleseNo ratings yet
- Equilibrium Phases of Pure Substance: Clapeyron EquationDocument4 pagesEquilibrium Phases of Pure Substance: Clapeyron EquationJksgNo ratings yet
- Thermodynamic Properties of FluidsDocument35 pagesThermodynamic Properties of FluidsYuli PeNo ratings yet
- Pressure and Thermodynamics FundamentalsDocument2 pagesPressure and Thermodynamics FundamentalsPearl Alexandra FabitoNo ratings yet
- Clausius-Clapeyron Equation ExplainedDocument37 pagesClausius-Clapeyron Equation ExplainedAkpotozor MarvisNo ratings yet
- Fluids 1Document35 pagesFluids 1Gurunath EpiliNo ratings yet
- Ideal Gas ProcessesDocument6 pagesIdeal Gas ProcessesKlydeJoseNo ratings yet
- 1 Law of Thermodynamics: Physical Chemistry I I Chemical ThermodynamicsDocument17 pages1 Law of Thermodynamics: Physical Chemistry I I Chemical Thermodynamicsetsegenet lakewNo ratings yet
- Volumetric Properties of Pure FluidsDocument19 pagesVolumetric Properties of Pure FluidsgNo ratings yet
- Homework Week 5: 3 Ext Ext 3 ExtDocument10 pagesHomework Week 5: 3 Ext Ext 3 ExtIsabelle SimãoNo ratings yet
- Joule Thomson EffectDocument18 pagesJoule Thomson EffectEdmond YuenNo ratings yet
- Chapter 4 - Fugacity - +ChemPot2011 - AnnotatedDocument46 pagesChapter 4 - Fugacity - +ChemPot2011 - AnnotatedMaruthappan SundaramNo ratings yet
- ME3322C Thermodynamics: Entropy Balance For A Control VolumeDocument9 pagesME3322C Thermodynamics: Entropy Balance For A Control Volumefqnguyen8No ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- Gaseous State Worksheet PDFDocument10 pagesGaseous State Worksheet PDFHarsh Agarwal100% (1)
- Property Table From Direct Measurement Equation of State Any Equations That Relates P, V, and T of A SubstanceDocument52 pagesProperty Table From Direct Measurement Equation of State Any Equations That Relates P, V, and T of A SubstanceKamran Mostajiri100% (1)
- Thermodynamics 1: PVT Properties of Pure FluidsDocument11 pagesThermodynamics 1: PVT Properties of Pure FluidsHabib Faisal YahyaNo ratings yet
- Experiment 6 Vapor Pressure of Pure LiquidsDocument8 pagesExperiment 6 Vapor Pressure of Pure LiquidsHaNo ratings yet
- GATE AEROSPACE Engineering Compressible Fluid FlowDocument11 pagesGATE AEROSPACE Engineering Compressible Fluid FlowAshok KumarNo ratings yet
- Thermodynamic Process Equations and DiagramsDocument10 pagesThermodynamic Process Equations and DiagramsAbenayaNo ratings yet
- Thermodynamic Potentials Unit3Document19 pagesThermodynamic Potentials Unit3Anonymous odl3MBNo ratings yet
- Introduction To Compressible Fluid FlowsDocument44 pagesIntroduction To Compressible Fluid FlowsShahzada ShujaNo ratings yet
- Du Tds PDV DH Tds VDP Da SDT PDV DG SDT VDP: Chapter 11 SummaryDocument4 pagesDu Tds PDV DH Tds VDP Da SDT PDV DG SDT VDP: Chapter 11 SummaryGitanjali TomarNo ratings yet
- Chemical Engineering Thermodynamics Property RelationsDocument70 pagesChemical Engineering Thermodynamics Property RelationsApple EmiratessNo ratings yet
- CH 2 PDFDocument34 pagesCH 2 PDFkrishnaNo ratings yet
- Notes in Compressible Uid Ow: April 2019Document15 pagesNotes in Compressible Uid Ow: April 2019Renata RamosNo ratings yet
- Compressibility PDFDocument2 pagesCompressibility PDFJuan Daniel CabreraNo ratings yet
- Thermodynamic Property RelationsDocument51 pagesThermodynamic Property RelationsBilal AhmedNo ratings yet
- Lec 3Document11 pagesLec 3조기현/초빙교수/스마트소재부품공학No ratings yet
- Property relations and equations for homogeneous phasesDocument20 pagesProperty relations and equations for homogeneous phasesAbdur RehmanNo ratings yet
- Ch.1,214-FOU 5Document84 pagesCh.1,214-FOU 5Ahmed YounisNo ratings yet
- Thermodynamics and Entropy Eng ItaDocument40 pagesThermodynamics and Entropy Eng ItaLeonardo RubinoNo ratings yet
- Thermodynamics Laws EquationsDocument2 pagesThermodynamics Laws EquationsPreston KendallNo ratings yet
- Koretsky Chapter 5 Engineering and Chemical ThermodynamicsDocument81 pagesKoretsky Chapter 5 Engineering and Chemical ThermodynamicsNidia MfNo ratings yet
- 10 Most Important Derivations With SolutionsDocument18 pages10 Most Important Derivations With SolutionsManish PattajoshiNo ratings yet
- Solution To Statistical Physics Exam: 29th June 2015Document13 pagesSolution To Statistical Physics Exam: 29th June 2015*83*22*No ratings yet
- EntropyDocument20 pagesEntropykurakid100% (2)
- The Effect of Applied Pressure On Vapor Pressure:) (M, Equilibriu atDocument20 pagesThe Effect of Applied Pressure On Vapor Pressure:) (M, Equilibriu atVeliyana Londong AlloNo ratings yet
- Properties of Pure Substance - Spring 2020Document83 pagesProperties of Pure Substance - Spring 2020Hamza AliNo ratings yet
- EU2-Chap 4Document2 pagesEU2-Chap 4Kevin Mark IlaganNo ratings yet
- 1st Law of ThermodynamicsDocument20 pages1st Law of ThermodynamicsS2 MelodyNo ratings yet
- 2.gaseous State PDFDocument13 pages2.gaseous State PDFP. E. I. AcademicsNo ratings yet
- Chapter 4Document20 pagesChapter 4DertySulistyowatiNo ratings yet
- Ideal GasDocument24 pagesIdeal Gastechno studioNo ratings yet
- DILL CH - 14 Phase EquilibriumDocument18 pagesDILL CH - 14 Phase EquilibriumMohamed MahmoudKhattabNo ratings yet
- Lecture Five 2 Per PageDocument30 pagesLecture Five 2 Per PagewardraNo ratings yet
- Thermodynamics - Cheat SheetDocument2 pagesThermodynamics - Cheat SheetJonathan0% (1)
- GASEOUS STATEDocument4 pagesGASEOUS STATEGaganNo ratings yet
- 9-Chapter 8-Chemical Reaction Equilibria-27March Online Class-STDNDocument15 pages9-Chapter 8-Chemical Reaction Equilibria-27March Online Class-STDNMahamed HusseinNo ratings yet
- Colloid Chemistry: Effect of Radius on EquilibriumDocument20 pagesColloid Chemistry: Effect of Radius on EquilibriumBasketball LoverNo ratings yet
- 化工热力学补充习题(英文)解析Document14 pages化工热力学补充习题(英文)解析Vyan IlhamNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Seismic load analysis for X and Y directionsDocument4 pagesSeismic load analysis for X and Y directionsJeo CandilNo ratings yet
- DPM Class-NotesDocument38 pagesDPM Class-NotesZephaniah MuneneNo ratings yet
- Arya A.P. Introduction To Classical Mechanics (2ed., PH, 199Document718 pagesArya A.P. Introduction To Classical Mechanics (2ed., PH, 199andifisika100% (4)
- Steel Truss Bridge Design ExampleDocument63 pagesSteel Truss Bridge Design ExampleCarlos Silva Castillo75% (4)
- 3 Hinged ArchsDocument9 pages3 Hinged ArchsFi FaNo ratings yet
- Transport PhenomenaDocument6 pagesTransport PhenomenaMITZINo ratings yet
- Practise Test On Physics by Yomal AmarathungeDocument3 pagesPractise Test On Physics by Yomal AmarathungeYomal AmarathungeNo ratings yet
- Industrial/Power Plant Engineering: Prepared By: Engr. Jose R. FranciscoDocument6 pagesIndustrial/Power Plant Engineering: Prepared By: Engr. Jose R. FranciscoJerick HernandezNo ratings yet
- Bonfiglioli Cafs Hajtomu KatalogusDocument538 pagesBonfiglioli Cafs Hajtomu Katalogussanjeev100% (2)
- 1ST Mid Term Form 4 2020 (Answer)Document4 pages1ST Mid Term Form 4 2020 (Answer)Nurul SalwanaNo ratings yet
- Static Pressure Calculator GuideDocument6 pagesStatic Pressure Calculator GuideNghiaNo ratings yet
- AHU IsolationDocument1 pageAHU IsolationNandkumar PatilNo ratings yet
- DNV Marine Operation Rules For Subsea LiftingDocument48 pagesDNV Marine Operation Rules For Subsea LiftingdrailotaNo ratings yet
- Physics RG 5.2 Answer KeyDocument3 pagesPhysics RG 5.2 Answer KeymrgrindallNo ratings yet
- DnsFoam Tutorial Martin de Mare v3Document15 pagesDnsFoam Tutorial Martin de Mare v3rakendreddyNo ratings yet
- Lacture # (Week-01) Fluid Mechanics-IIDocument26 pagesLacture # (Week-01) Fluid Mechanics-IIMushaf KhalidNo ratings yet
- MEB3023 - Lecture2 (A) - V Belt DriversDocument39 pagesMEB3023 - Lecture2 (A) - V Belt DriversRoger QinNo ratings yet
- Impulse Turbine and Reaction Turbine-Principle, Working and Difference - Mechanical Engineering Site PDFDocument10 pagesImpulse Turbine and Reaction Turbine-Principle, Working and Difference - Mechanical Engineering Site PDFpavijaya100% (1)
- Advanced Nuclear Physics by Imran AzizDocument187 pagesAdvanced Nuclear Physics by Imran AzizDr.Imran AzizNo ratings yet
- Ramiro Augusto Salazar La Rotta, Ing. Químico, PH.D., CC: 91227727 Colombia (UIS, SENA) Juan Manuel Salazar Villamizar (Hijo) - E-Mail:, AbstracDocument7 pagesRamiro Augusto Salazar La Rotta, Ing. Químico, PH.D., CC: 91227727 Colombia (UIS, SENA) Juan Manuel Salazar Villamizar (Hijo) - E-Mail:, AbstracRamiro Augusto salazar La RottaNo ratings yet
- Land Reclamation Proposal SummaryDocument11 pagesLand Reclamation Proposal Summaryprasadnn2001No ratings yet
- Aristotle Vs Galileo MotionDocument5 pagesAristotle Vs Galileo MotionNathan John PaguiriganNo ratings yet
- Design of Steel HangerDocument2 pagesDesign of Steel HangerMostafa Saleh100% (1)
- Modeling of Buried Natural Gas Pipeline Decompression: X. L. Zhou G. G. KingDocument8 pagesModeling of Buried Natural Gas Pipeline Decompression: X. L. Zhou G. G. Kingmatrix69No ratings yet
- Allowable Stresses of Phil WoodDocument2 pagesAllowable Stresses of Phil WoodTos Hernando100% (2)
- (Why We Need) An Operations Paradigm For ServicesDocument42 pages(Why We Need) An Operations Paradigm For ServicesdeepaksinghmbaNo ratings yet
- Tunnelling and Underground Space TechnologyDocument11 pagesTunnelling and Underground Space TechnologyHaha ZazaNo ratings yet
- 4 and 5th Lesson MCQDocument5 pages4 and 5th Lesson MCQDhanush RamanNo ratings yet
- Question Text: One Sack of RiceDocument102 pagesQuestion Text: One Sack of RiceJelyn Ramirez67% (3)
- Nastran in Cad 2019 1 Essentials PDFDocument44 pagesNastran in Cad 2019 1 Essentials PDFThierry OUAMBO FOTSO0% (1)