Professional Documents
Culture Documents
CHM2010 1
CHM2010 1
Uploaded by
DanielaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHM2010 1
CHM2010 1
Uploaded by
DanielaCopyright:
Available Formats
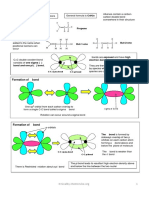
chapter one: carbon compounds and chemical bonds
-
convert to lewis structure
:O :
-0 ALWAYS !
11 . .
H -
C -
H -
H -
not allowed to
change order of atoms
-
:O :
change single bonds
•
µ _ ¥ÑH @ •
-
allowed to move
mlannot
pi bonds ltelond bone of double bond )
I
-
move
Milan more lone pairs
'
:O :-O
start from electron sink
moving
__
-
i
H -
l=Ñ -
H
Earless of electrons positive Marge
to electron sink electrons
or
missing
resonance
8-1
hybrid : -
donotpvttvll charge
:& g.
on resonance
hybrid
On resonance
Ni
-
H -
H l -
* the correct way to partial charge
◦
show resonant •
partial bond
structure
•
lone pairs wl
double bond
You might also like
- Structure of Atom Shobhit NirwanDocument15 pagesStructure of Atom Shobhit NirwanBhavya Goyal XI Non med92% (12)
- Carbon and Its Compounds Shobhit NirwanDocument9 pagesCarbon and Its Compounds Shobhit NirwanRishabh Joshi78% (9)
- Strain MeasurementDocument40 pagesStrain MeasurementNavinRajSakaranNo ratings yet
- Report No. 1 - PROPELLER CONSTRUCTIONDocument10 pagesReport No. 1 - PROPELLER CONSTRUCTIONRev Xavier CruzNo ratings yet
- LHHW KineticsDocument3 pagesLHHW KineticsDanny Nguyen67% (3)
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular StructureRao GootleyNo ratings yet
- 3 Sigma and PaiDocument1 page3 Sigma and PaikalloliNo ratings yet
- Concise OrganicDocument2 pagesConcise Organic4k.danieldiscordNo ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular Structuresarthakyedlawar04No ratings yet
- 10april ITCM921E01Document10 pages10april ITCM921E01NanaiNo ratings yet
- Basic Organic Chemistry-MergedDocument256 pagesBasic Organic Chemistry-MergedYash SinghNo ratings yet
- Molecules To KnowDocument31 pagesMolecules To KnowFedeNo ratings yet
- Adobe Scan 12-Dec-2022Document1 pageAdobe Scan 12-Dec-2022Kushagra AgrawalNo ratings yet
- Structure of Atom FinalDocument15 pagesStructure of Atom FinalAbhinavNo ratings yet
- General Chemistry 2Document20 pagesGeneral Chemistry 2Pauline Jade TrespecesNo ratings yet
- Chemical Bonding One Day One Chapter Nitesh DevnaniDocument41 pagesChemical Bonding One Day One Chapter Nitesh Devnanivrinda11xxNo ratings yet
- 5 Alkenes Iedxcel779Document7 pages5 Alkenes Iedxcel779Best ProgressNo ratings yet
- Together: I of BindDocument15 pagesTogether: I of BindShreyas PrabhuNo ratings yet
- Semiconductor and DiodesDocument19 pagesSemiconductor and Diodesf20230485No ratings yet
- Che 118 ADocument16 pagesChe 118 Aapi-515370734No ratings yet
- Hydrogen - BrahmastraDocument46 pagesHydrogen - BrahmastraStevensonNo ratings yet
- St-ES: Wao.cDocument18 pagesSt-ES: Wao.cWilliam WangNo ratings yet
- Instant: The UDocument52 pagesInstant: The UTushar BhardwajNo ratings yet
- 1.1 Lewis Structure & VSEPRDocument11 pages1.1 Lewis Structure & VSEPRNurul Izzah KaharNo ratings yet
- Rigidity of Double /triple Bonds: C H H H H H C H H H H C H H H CDocument7 pagesRigidity of Double /triple Bonds: C H H H H H C H H H H C H H H CkalloliNo ratings yet
- Chapter 8 AssessmentDocument19 pagesChapter 8 AssessmentLeinNo ratings yet
- IB BondingDocument12 pagesIB BondingLetso JamesNo ratings yet
- ChimieDocument5 pagesChimieAlexandru RoinitaNo ratings yet
- 08 Atom NucleiDocument17 pages08 Atom NucleiPreet KaurNo ratings yet
- CommonlyTestedQns PhysicalChemistryDocument7 pagesCommonlyTestedQns PhysicalChemistryAnarkin FitriNo ratings yet
- Inorganic As LevelDocument63 pagesInorganic As Levelchan chanNo ratings yet
- Wagner-Meerwein RearrangementsDocument2 pagesWagner-Meerwein RearrangementsNguyễn Đình SơnNo ratings yet
- Chemical BondingDocument11 pagesChemical Bondingvenom eNo ratings yet
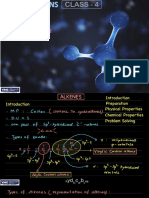
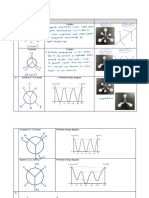
- Hydrocarbons Live Class-4 Teacher NotesDocument28 pagesHydrocarbons Live Class-4 Teacher Notesayushgenii1055No ratings yet
- Copy of Manual Amali Sko3023 Sem A222Document5 pagesCopy of Manual Amali Sko3023 Sem A222NURUL ZAKIRAH BINTI BORHANUDINNo ratings yet
- Copy of MANUAL AMALI SKO3023 SEM A222Document5 pagesCopy of MANUAL AMALI SKO3023 SEM A222NURUL ZAKIRAH BINTI BORHANUDINNo ratings yet
- Cabanis + Lecolle 1989Document1 pageCabanis + Lecolle 1989benjamin pNo ratings yet
- EE2004 Fundamentals of Circuits: Lecture 2: Voltage, Current, Power Michael KnoxDocument29 pagesEE2004 Fundamentals of Circuits: Lecture 2: Voltage, Current, Power Michael KnoxVera SunNo ratings yet
- Goc and Isomerism 2 d95EXDjeXNFkPn68Document12 pagesGoc and Isomerism 2 d95EXDjeXNFkPn68PRANJUL SAHUNo ratings yet
- Adobe Scan Jan 29, 2024Document5 pagesAdobe Scan Jan 29, 2024dericksonj32No ratings yet
- Carbon and Its Compounds - Class - 10Document9 pagesCarbon and Its Compounds - Class - 10Mamta JoshiNo ratings yet
- Stereoselective Reactions of Alkenes: Single Diastereoisomers Pre-Existing StereogenicDocument25 pagesStereoselective Reactions of Alkenes: Single Diastereoisomers Pre-Existing StereogenicSubhabrata MabhaiNo ratings yet
- Draw Lewis STR Predicting Shape & Plarity & Hybridisation EditedDocument4 pagesDraw Lewis STR Predicting Shape & Plarity & Hybridisation EditedDurga ShrieNo ratings yet
- 03 Nervous System ADocument116 pages03 Nervous System ABalew KassieNo ratings yet
- عضويةDocument12 pagesعضويةأ. علي محمدNo ratings yet
- Carbon and Its Compounds VerifiedDocument10 pagesCarbon and Its Compounds VerifiedLimitless VoidNo ratings yet
- NoteDocument1 pageNote25khanhnhuNo ratings yet
- Imaginary: AcquiringDocument20 pagesImaginary: AcquiringCat123No ratings yet
- PharmacophorePatterns PDFDocument29 pagesPharmacophorePatterns PDFIoana Mirela VasincuNo ratings yet
- 3 Chapter9-10Document69 pages3 Chapter9-10Ikushou SoNo ratings yet
- Kab SCH 1983Document61 pagesKab SCH 1983AsterasNo ratings yet
- Ligand Exchange Mechanisms: M.C. White, Chem 153 Mechanism - 43-Week of September 24th, 2002Document42 pagesLigand Exchange Mechanisms: M.C. White, Chem 153 Mechanism - 43-Week of September 24th, 2002ririsarista14No ratings yet
- 3 4 Revision Guide AlkenesDocument4 pages3 4 Revision Guide AlkenesNandiNo ratings yet
- Xtra SaltDocument2 pagesXtra SaltAisyah Nur PutriNo ratings yet
- Goc FS9Document133 pagesGoc FS9mannangupta83No ratings yet
- Circuit Class Note PDFDocument99 pagesCircuit Class Note PDFmaxwell J.P.No ratings yet
- Materials Science and Engineering: Chapter - 04Document29 pagesMaterials Science and Engineering: Chapter - 04박남주No ratings yet
- Alkenes: H H H HDocument13 pagesAlkenes: H H H Hhamda yNo ratings yet
- Dmpetant Qushons: Module-1 NbvbonDocument2 pagesDmpetant Qushons: Module-1 NbvbonRajesh K MNo ratings yet
- 1 Semester Organic Chemistry Now in 1 % The Time!Document23 pages1 Semester Organic Chemistry Now in 1 % The Time!Tùng SuNo ratings yet
- MIT20 441JF09 Lec08 Ms PDFDocument20 pagesMIT20 441JF09 Lec08 Ms PDFlotannaNo ratings yet
- SemiconductorDocument22 pagesSemiconductorsatorugojosensei4189No ratings yet
- Sf8010 Pavement Engineering L T P CDocument6 pagesSf8010 Pavement Engineering L T P CRavichandran.PNo ratings yet
- HeatDocument10 pagesHeatJom BonhayagNo ratings yet
- Relationship Elastic Constants: BetweenDocument19 pagesRelationship Elastic Constants: BetweenDr. BIBIN CHIDAMBARANATHANNo ratings yet
- Huawei - SUN2000 17KTL M2 DatasheetDocument2 pagesHuawei - SUN2000 17KTL M2 DatasheetNhacaNo ratings yet
- Tutorial 1Document5 pagesTutorial 1Shaon SenNo ratings yet
- Ficha Técnica Hdpe (Sabic)Document2 pagesFicha Técnica Hdpe (Sabic)Luis LiraNo ratings yet
- Mechanical Properties of Materials ADocument3 pagesMechanical Properties of Materials ATIgist MelkamNo ratings yet
- Chap 4. SuperconductorDocument8 pagesChap 4. SuperconductorAbu Talha TalhaNo ratings yet
- DivyaDocument34 pagesDivyaAnonymous 0O7Xpz90% (1)
- An Intermediate Heating and Cooling Method For A Distillation ColumnDocument7 pagesAn Intermediate Heating and Cooling Method For A Distillation ColumnAnonymous N3LpAXNo ratings yet
- Stronger in Compression Than in TensionDocument4 pagesStronger in Compression Than in TensionMohamed MagedNo ratings yet
- Lecture 2 Theory of PlasticityDocument16 pagesLecture 2 Theory of PlasticityprashanthattiNo ratings yet
- Polystone 7000 Product Information Rev.1 27-8-19Document1 pagePolystone 7000 Product Information Rev.1 27-8-19Dan MoldoveanuNo ratings yet
- Unit-2 QuestionsDocument3 pagesUnit-2 Questionsabhioptimus00No ratings yet
- ProjectDocument2 pagesProjectKRISHNA DAVENo ratings yet
- EMM Lab ManualDocument4 pagesEMM Lab Manualbhajneets2005100% (1)
- D and F Block PPT NCERT Line by LineDocument512 pagesD and F Block PPT NCERT Line by Lineafaqahmed7019No ratings yet
- Design of Rigid Pavement For Industrial Focal Point NabhaDocument4 pagesDesign of Rigid Pavement For Industrial Focal Point NabhaAnonymous ciKyr0tNo ratings yet
- LED TelevisionDocument12 pagesLED Televisiondevilr796No ratings yet
- Cold Spray PTFEDocument4 pagesCold Spray PTFEKewell LimNo ratings yet
- A 182Document20 pagesA 182Thomas100% (1)
- Cold Mounting Systems For All Materialographic ApplicationsDocument6 pagesCold Mounting Systems For All Materialographic ApplicationsDEWI SRIREJEKI LESTARI -No ratings yet
- The Effect of Sea Water Treatment On The Impact AnDocument6 pagesThe Effect of Sea Water Treatment On The Impact AnFathony 7No ratings yet
- An Overview of Recent Studies On The Analysis of Pharmaceutical PolymorphDocument27 pagesAn Overview of Recent Studies On The Analysis of Pharmaceutical Polymorphrafael_nicolay9196No ratings yet
- Seminar On Harden ConcreteDocument24 pagesSeminar On Harden ConcreteJatin ChaudhariNo ratings yet
- EE1 LecturenotesDocument53 pagesEE1 LecturenotesVivek SuranaNo ratings yet
- Alignment Chart For Effective Length of Columns in Continuous FramesDocument1 pageAlignment Chart For Effective Length of Columns in Continuous Framesivan bolañosNo ratings yet