Professional Documents
Culture Documents
ETP 4 Essay Marking Part B, C
Uploaded by
CU GOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ETP 4 Essay Marking Part B, C
Uploaded by
CU GCopyright:
Available Formats
AL/2022/02/S-III
2022 – Exam Target Paper 04 (Essay) - Marking Scheme
B fldgi - rpkd
5.
(a)
(i)
I. ∆HR∅ = ∑ ∆Hf∅ (M,) − ∑ ∆Hf∅ (m%;sl%Shl) (02)
= (− 242 kJmol−1 × 3) − (− 822 kJmol−1 ) (03)
= 96 kJmol−1 (03)
∆SR∅ = ∑ ∆S ∅ (M,) − ∑ ∆S ∅ (m%;sl%Shl) (02)
= (2 × 27 Jmol K −1 −1 )
+ (3 × 189 Jmol−1 K −1 ) (03)
= 138 Jmol−1 K −1 (03)
300 K oS, ∆G = ∆H − T∆S (03)
= 96 kJmol −1
− 300 K × 0.138 kJmol−1 K −1 (03)
= + 54.6 kJmol−1 (2 + 1)
∴ 300 K oS m%;sl%shdj iajhxisoaO fkdfõ' (02)
800 K oS, ∆G = ∆H − T∆S
= 96 kJmol−1 − 800 K × 0.138 kJmol−1 K −1 (03)
= − 14.4 kJmol −1 (2 + 1)
∴ 800 K oS m%;sl%shdj iajhxisoaO fõ' (02)
II. WIaK;ajh u; tka;e,ams w.hka yd tkafg%dms w.hka fjkia fkdjk nj' (05)
(ii)
I. jeä fõ'
II. fjkia fkdfõ'
III. wvq fõ' (5 × 3 = 15)
(b)
a b
(i) R = k [P(aq) ] [Q (aq) ] (10)
WIaK;ajh u; r|d mj;sk : R − m%;sl%shd YS>%;dj'
: K − YS>%;d ksh;h' (1 × 2 = 2)
WIaK;ajh u; r|d fkdmj;sk : a − P g idfmalaIj fm<'
: b − Q g idfmalaIj fm<' (1 × 2 = 2)
(ii) P(aq) + Q (aq) R (aq) + S(aq) hk m%;sl%shdj uQ,l
s m%;sl%shdjla ùu' (06)
(iii) m%;sl%shd YS>%;dj Q m%udKh u; r|d fkdmj;sk neúka Q g idfmalaIj fm< 0 úh hq;=h' (03)
∴ b=0
a
R = k [P(aq) ] (05)
a
R ∝ [P(aq) ] (02)
2.4 × 10−5 moldm−3 s −1 ∝ [2 × 10−2 moldm−3 ]a 1
4.8 × 10−5 moldm−3 s −1 ∝ [4 × 10−2 moldm−3 ]a 2 (5 × 2 = 10)
CHEMISTRY 5 Amila Dasanayake
AL/2022/02/S-III
a
2⁄ ⟹ 4.8 × 10−5 4 × 10−2
1 2.4 × 10−5
=(
2 × 10−2
) (02)
2 = 2a
a=1 (03)
∴ P g idfmalaIj fm< 1 ls'
iuia; fm< = a + b = 1 + 0 = 1 (05)
(iv) All (05)
(c)
dRT
(i) P = (02)
M
dRT
ñY%Kfha uOHkh ujq,sl ialkaOh (M) =
P
1×10−2 ×103 kgm−3 × 8.314 Jmol−1 K−1 × 400K
M=
8.314×105 Nm−2
(02)
M = 40 g (02)
2 O3 (g) 3 O2 (g)
wdrïNl 4 mol
m%;sl%shd jk' −2x +3x mol
iu;=,;
s 4 − 2x +3x mol (02)
(4−2𝑥) 48 + 3𝑥 × 32
40 =
4+𝑥
192 − 96𝑥 + 96𝑥
40 =
4+𝑥
𝑥 = 0.8 mol (02)
fvda,agka wdxYsl mSvk kshufhka,
PO3 = Ptot × X O3 (02)
(4−2×0.8) mol
= 8.314 × 105 Nm−2 × (02)
(4+0.) mol
= 4.157 × 105 Nm−2 (03)
3
[O2 (g) ]
(ii) K C = 2
[O3 (g) ]
P3
O 2 (g)
KP =
P2
(2 × 2 = 4)
O 3 (g)
(iii) PV = nRT
nRT
P=
V
P = CRT (04)
P3
O 2 (g)
KP =
P2
O 3 (g)
3
([O2 (g) ] RT)
KP = 2 (02)
([O3 (g) ] RT)
K P = K C RT (04)
CHEMISTRY 6 Amila Dasanayake
AL/2022/02/S-III
PO2 = Ptot × X O2
= 8.314 × 105 Nm−2 × 0.5
= 4.157 × 105 Pa (02)
3
(4.157×105 Pa )
KP = (4.157×105 Pa )2
= 4.157 × 105 Pa (02)
KP
KC =
RT
4.157×105 Pa
= (02)
8.314 Jmol−1 K−1 × 400K
= 125 molm−3 (2 + 1)
6.
(a)
(i) HA(aq) A−(aq) + +
H(aq)
(05)
iu;=,;
s 0.2 − 𝑥 𝑥 𝑥 moldm−3
+
[A−
(aq) ] [H(aq) ]
Ka =
[HA(aq) ]
(03)
+
pH = − lg [H(aq) ] (02)
pH = 4.3 neúka, +
[H(aq) ] = 5 × 10−5 moldm−3 (02)
2
[5×10−5 moldm−3 ]
Ka = (0.2−𝑎) moldm−3
(3 + 2)
HA ÿn, wï,hla neúka, 0.2 − 𝑎 ⋍ 0.2
25×10−10
∴ Ka = moldm−3
0.2
= 125 × 10−10 moldm−3
= 1.25 × 10−8 moldm−3 (3 + 2)
(ii)
(I) pH = 5 neúka, [H(aq)
+
] = 1 × 10−5 moldm−3 (02)
2
[1×10−5 moldm−3 ]
1.25 × 10−8 moldm−3 =
[HA(aq) ]
1×10−10
[HA(aq) ] = moldm−3
1.25×10−8
= 8 × 10−3 moldm−3
= 0.008 moldm−3 (3 + 2)
0.2−0.008
CHCl3 l,dmhg .uka l, HA mol .Kk = × 200
1000
0.192
= mol
5
= 0.0384 mol
0.0384 mol
∴ [HA(CHCl3) ] = × 1000
100
= 0.384 moldm−3 (02)
[HA(CHCl3 ) ]
∴ KD = (03)
[HA(aq) ]
0.384 moldm−3
=
0.008 moldm−3
= 48 (05)
CHEMISTRY 7 Amila Dasanayake
AL/2022/02/S-III
(II) pH = 5.3 hkq, [H(aq)
+
] = 5 × 10−6 moldm−3
2
(5×10−6 moldm−3 )
1.25 × 10−8 moldm−3 =
[HA(aq) ]
25×10−12
[HA (aq) ] =
125×10−10
= 0.2 × 10−2 moldm−3
= 0.002 moldm−3 (03)
[HA(CHCl3 ) ]
KD = = 48
[HA(aq)]
[HA(CHCl3) ] = 48 × 0.002 moldm−3
= 0.096 moldm−3 (03)
0.2−0.002
CHCl3 l,dmhg .uka l, HA mol .Kk = × 100
1000
= 0.0198 mol
1000
∴ tla l, hq;= CHCl3 mßudj = × 0.0198
0.096
= 206.25 cm3 (3 + 2)
(iii)
(I) HA (aq) + NaOH(aq) H2 O(ℓ) + NaA (aq)
wdrïNl : 0.1 0.025 − − moldm−3
m%;sl%shdjk : − 0.025 − 0.025 − + 0.025 moldm−3
wjidk : 0.075 − − 0.025 moldm−3
(04)
[,jKh]
pH = pK a + lg (03)
[wï,h]
0.025
pH = 8 − lg 1.25 + lg
0.075
(03)
= 8 − lg 5 + lg 4 − lg 3
= 8 − 0.699 + 0.6021 − 0.4771
= 7.426 (05)
(II) [HA(aq) ] = C moldm−3 f,i .ksuq' ∴ [HA(CHCl3) ] = 48 C moldm−3
wdrïNl c,Sh HA mol .Kk = CHCl3 l,dmfha HA mol .Kk + c,Sh l,dmfha HA mol .Kk
0.15 × 100 × 10−3 = 48 C × 200 × 10−3 + C × 200 × 10−3 (03)
0.15 = 96 C + 2 C
0.15
C= moldm−3
98
C = 0.0015 moldm−3 (02)
∴ pH = pKa + lg [,jKh]
[wï,h]
[0.075]
= 7 + 0.903 + lg [0.0015]
= 7 + 0.903 + 0.699 + 1
= 9.6 (05)
CHEMISTRY 8 Amila Dasanayake
AL/2022/02/S-III
(b)
(i) NaOH (05)
wï, - NIau wkqudmk j,oS idudkHfhka ÿn, wï, fyda ÿn, NIau mj;S kï tajd wkqudmk ma,dial=jg
fhdohs' kuq;a fujeks m%n, wï, - m%n, NIau wkqudmk j, l=ula jqjo wkqudmk ma,dial=jg fhosh yel'
fuysoS Ndú; lrk ìhqfrÜgqj ùÿre WmlrKhla neúka NdIañl ødjK ta ;=< mej;Sfuka ydks úh yel'
tuksid NaOH wkqudmk ma,dial=jg oeóï jvd fhda.H fõ' (10)
úl,am ms<s;=re
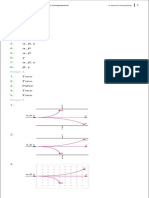
(ii) pH
pH
13
12
11 11
10 7
9 3
8
1
7
6 mßudj (cm3 )
5 iul;d
4 ,laIHh
3
2
1
(20)
mßudj (cm3 )
iul;d
,laIHh
(iii) 3 − 11 (05)
(iv) fuys wï,fha idkaøKh 1/10 jka neúka YS>% pH úp,Hh mrdifha wdï,sl ,laIHh 1 lska jeä fõ' (05)
túg pH mrdih 4 − 11 fõ' (05)
YS>% pH úp,Hhla mrdihla fkdue; hkakgo ms<s;=re ,ndfokak'
(v) (A) CH3 COOH (05)
(B) jeäúh hq;=h' (05)
CH3 COOH + NaOH CH3 COONa + H2 O
CH3 COONa CH3 COO− + Na+
Na X
−
CH3 COO + H2 O CH3 COOH + OH − (05)
fuu m%;sl%shdj wkqj fmfkkafka wka; ,laIHfha ødjKh NdIañl jk njhs' (05)
∴ iul;d ,laIHfha pH w.h 7 jeä úh hq;=h'
(c) *sfkd,ama;,Ska (05)
CHEMISTRY 9 Amila Dasanayake
AL/2022/02/S-III
7.
(a)
(i) E − [Fe(H2 O)4 ]2+ G − [Fe(H2 O)6 ]3+ (6 × 2 = 12)
(ii) A − K 3 [Fe(CN)6 ] C − NH4 [Fe(SCN)4 ]
B − K 4 [Fe(CN)6 ] D − H[FeCl4 ] (5 × 4 = 20)
(iii) tysoS ls,á
s fld< wjflaIamhla ,efnk w;r u| fj,djla jd;hg ksrdjrKh lr ;enQ úg r;a ÿUqre
wjlafYamhla ,efí' (10)
(iv) A − Potassium hexacyanoferrate(II)
B − Potassium hexacyanoferrate(III)
C − ammonium tetrathiocyanoferrate(III)
D − hydrogen tetrachloridoferrate(III) (5 × 4 = 20)
(b)
(i) P − (−) w.%h Q − (+) w.%h (2 × 2 = 4)
0.5 g
(ii) n Cu = = 8 × 10−3 mol (05)
64 gmol−1
Cu2+
(aq) + 2𝑒 Cu(s) (05)
n 𝑒 = n Cu × 2
= 8 × 10−3 mol × 2
= 16 × 10−3 mol (05)
Q = It (03)
Odrdj .eÆ ld,h t kï,
Q = (0.5 × t) C (03)
(0.5×t) C
.eÆ e’ k mol = (05)
96 500 C mol−1
(0.5×t)
16 × 10−3 mol = (05)
96 500
t = 3088 s (3 + 2)
(c)
(i) Mg (s) | Mg 2+ 3+ 2+
(aq ,1moldm−3 ) || Fe (aq ,1moldm−3 ) , Fe (aq ,1moldm−3 ) | Pt (s) (10)
(ii) Mg (s) Mg 2+
(aq) + 2𝑒 (05)
(iii) Fe3+
(aq) + 𝑒 Fe2+
(aq) (05)
(iv) Mg (s) + 2 Fe3+
(aq) Mg 2+ 2+
(aq) + 2 Fe (aq) (05)
(v) Ecell = Ecathode − Eanode (05)
= 0.77 V − (−2.37 V) (05)
= 3.14 V (3 + 2)
(vi) Odrdj .,k osYdj – lef;davfha isg wefkdavh foig' (05)
e’ k .,k osYdj – wefkdavfha isg lef;davh foig' (05)
CHEMISTRY 10 Amila Dasanayake
AL/2022/02/S-III
8.
(a)
(i) CH3 CH2 CHO
LiAlH4
(04)
H2 O
O
CH3 CH2 CH2 OH (04)
CH3 − C − CH3 (04) CH3 CH2 CH2 NH2 (04)
(04) PCl3 conc. H2 SO4 (04)
P. C. C (04)
dil. H2 SO4 conc. NH3 (04)
CH3 CH2 CH2 Cl CH3 CH2 = CH2 CH3 − CHOH − CH3
(04)
(04) (04) (04)
All correct (+1)
O CH = CH2
(b) −C− HO − − N2 −
NH2
aq. NaOH (2.5)
O
− C − O Na + − OH − OH − CH = CH2
(2.5) (2.5) Cl N2 (2.5)
NH2 (2.5)
H3 O+ (2.5)
NaNO2 / HCl
(2.5)
0−5℃
O
(2.5) − CH = CH2 (2.5)
− C − OH
NH2
NH2
LiAlH4 conc. H2 SO4 (2.5)
H2 O (2.5)
∆
− CH2 OH (2.5) − CH2 CH2 OH (2.5)
NH2 NH2
PCl5 (2.5) LiAlH4 (2.5)
H2 O
aq. alchoholic H3 O+
− CH2 Cl − CH2 CN − CH2 COOH
KCN (2.5)
(2.5) (2.5) (2.5)
NH2 (2.5) NH2 NH2
(50)
CHEMISTRY 11 Amila Dasanayake
AL/2022/02/S-III
(c) O
(i) CH3 CH2 − C − Cl (04) + CH3 NH2 (04)
O
CH3 CH2 − C − NH2 + CH3 Cl
(04) (04)
O
(ii) m%;sldrlh = CH3 CH2 − C − NH2 (4.5)
Q jdhqj = NH3 (4.5)
(d)
(i) bj;aùfï m%;sl%shd (05)
H H
(ii) H − C − C − Cl CH = CH2 + H2 O + Cl−
H H (05)
(05)
(10)
OH −
9.
(a)
(i) A ⟶ NiS [c part tfla Mg/Al ialkaO ^ujq,sl& oS ke;']
B ⟶ H2 S
C ⟶ [Ni(OH)6 ]2+ / Ni2+
D ⟶ Ni(OH)2
E ⟶ [Ni(NH3 )6 ]2+ (5 × 5 = 25)
(ii) NiS(s) + H2 SO4 (aq) NiSO4 (aq) + H2 S(g) (10)
(iii) H2 S S + 2H + + 2e
14 H + + Cr2 O7 2− + 6e 2Cr 3+ + 7H2 O
3 H2 S + 8 H + + Cr2 O7 2− 2 Cr 3+ + 3S + 7 H2 O (5 × 3 = 15)
(b)
(i) NaI (10)
(ii) 1 − 6H + + IO3− + 5I − 3I2 + 3H2 O (10)
2 − 2I − + 2Cu2+ 2Cu+ + I2 (05)
I− I− (05)
3 − 2Na 2 S2 O3 + I2 2NaI + Na 2 S4 O6 (10)
(iii) >k wjia:dfõ oe,sia f,i mej;Su' (10)
(c)
(i) Mg + NaOH X
Cu + 2NaOH Cu(OH)2 + 2Na+
2Al + 2NaOH + 2H2 O 2NaAlO2 + 3H2
Mg + 2HCl MgCl2 + H2 (2.5 × 4 = 10)
CHEMISTRY 12 Amila Dasanayake
AL/2022/02/S-III
1
(ii) uq,a wjia:dfõ msg jQ H2 jdhq mol = × 672 = 0.03 mol (05)
22400
1
fojk wjia:dfõ msg jQ H2 jdhq mol =
22400
× 224 = 0.01 mol (05)
0.03
∴ mej;s Al mol =
2
× 2 = 0.02mol (05)
Al ialkaOh = 0.02 × 27 = 0.54g (05)
0.54 540
Al % = × 100% = = 45% (05)
1.2 12
∴ mej;s Mg mol = 0.01 mol (05)
Mg ialkaOh = 0.01 × 24 = 0.24g (05)
0.24 240
Mg % = × 100% = = 20% (05)
1.2 12
Mg , Cu , Al muKla iys; neúka
Cu = 100 − (20 + 45)% = 35% (05)
10.
(a)
(i) i,a*¾ fyda i,a*¾ wvx.= f,damia
jd;h
c,h (𝟐 × 𝟑 = 𝟔)
(ii) i,a*¾ fyda i,a*¾ wvx.= f,damia jd;fha oykh lsÍfuka SO2 jdhqj ,nd .kshs' (02)
S(s) + O2(g) SO2(g) (03)
tu ksmojd .;a SO2 jdhqj W;afm%rl yd jeämqr Tlaiscka iu. 450°𝐶 oS m%;sl%shd lrjd SO3 ,nd .kshs' (02)
𝑉2 𝑂5
2 𝑆𝑂2(𝑔) + 𝑂2 2 𝑆𝑂3(𝑔) (03) ∆H = (−)
450 ℃ − 500 ℃
1 𝑎𝑡𝑚 (;;a;aj + 4)
SO3 jdhqj 98% idkaø H2 SO4 j,g wjfYdaIKh lrjd iOQu i,a*shqßla fyj;a T,shï idod.kS' (02)
SO3(g) + H2 SO4(l) H2 S2 O7(l) (03)
T,shï m%fõYfuka c,h yd ñY% lr idkaø 𝐻2 𝑆𝑂4 wï,h ksmojhs' (02)
H2 S2 O7(l) + H2 O 2H2 SO4 (03)
(iii) SO2 , SO3 njg m;aùfï m%;sl%shdj ;dmodhl jk neúka my< WIaK;aj Bg ys;lr fõ' kuq;a WIaK;ajh
b;d wvq l<fyd;a m%;sl%shd iS>%;dj wvqùfuka iu;=,;
s ;djhg t<öug .;jk ld,h jeä fõ' tu ksid
m%Yia; WIaK;ajh f,i 450 ℃ − 500 ℃ Ndú; fõ' (05)
SO2 fyda O2 jeämqr fhoSfuka m%;sl%shdj bosßhg keUqre l< yel' SO2 : O2 = 1 ∶ 1 f,i mj;ajd .kS' Bg
jvd O2 j,ska ix;Dma; ù SO2 jdhqj wêfYdaIKh ùug we;s bvlv wvqùfuka m%;sl%shd iS>%;dj wvqùug
fya;= fõ' (05)
by< mSvk fhoSfuka m%;sl%shdj bosßhg keUqre fõ' kuq;a jdhqf.da,Sh mSvkfhaoS 99% ld¾hlaIu;djhla
we;s lsÍu ksid wu;r mSvkhla jeä fkdlrhs' (05)
(iv) jdhq j¾. úh<Sug'
vhsj¾., mqmr
q k øjH yd T!IO ksIamdokhg'
Bhï welshqñf,agr j, úoahq;a úÉfÊoHh f,i'
;Ska; j¾., lD;su fl|s, ma,diaála j¾. ksIamdokh' (𝟐. 𝟓 × 𝟐 = 𝟓)
CHEMISTRY 13 Amila Dasanayake
AL/2022/02/S-III
(b)
(i) Ydl yd ;;a;aj foay fldgia j, wvx.= jdIamYS,S wdfõKsl iqj|la we;s f;,a j¾. fõ' (04)
(ii) yqud, wdijkh'
ødjl ksiaidrKh'
f;rmSu' (𝟐 × 𝟑 = 𝟔)
(iii) f;rmSu (05)
wjdis – ,efnk M, m%udKh wvqùu'
f;,a fjk;a ldnksl øjH iuÕ ñY% ùu' (𝟐. 𝟓 × 𝟐 = 𝟓)
lrouqx.= îc
(iv) a. fmrksfhda,a
b. iskue,aäyhsâ
c. leïm¾ (𝟐 × 𝟑 = 𝟔)
(c)
(i) iQ¾h lsrK yuqfõ mßir ¥Il ldrl lSmhla (NO, jdIamYS,S yhsfv%dldnk) 15 ℃ g jeä WIaK;ajfhaoS
tlsfkl m%;sl%shd lr we;sjk ridhksl øjH, ishqï wxY= yd c, ì|s;s uÕska iQ¾h lsrK m%;srKh ùfuka
jdhqf.da,fha mdroDYH;djh wvqùuhs' (15)
(ii) jdyk ;onoh wêl k.rhl wmrNd.fha oS f.dvke.s,s j,g ;rula by<ska neÆ úg mdroDYH;dj wvq
ÿUqre meye;s ;sñr mg,hla oel.ekSug yel' (10)
(iii) PAN
PBN
jdIamYS,S we,aäyhsâ
O3 (𝟑 × 𝟒 = 𝟏𝟐)
2 NO(g) + O2 (g) 2 NO2 (g)
NO2 (g) h H NO(g) + O(g)
O°(g) + O2 (g) O3 (g) (𝟐 × 𝟑 = 𝟔) ´fidaka iEoSu (02)
(iv) ´fidaka uÕska j¾Kl úrxckh fõ' frosms<s j, .=Kd;aul nj wvqfõ'
´fidaka úI jdhqjls' th wd>%dKh ùfuka Yajik wdndO we;sfõ'
PAN yd PBN cdk úlD;s;d we;s lrhs'
jdIamYS,S we,aäyhsâ wd>%dKfhka Yajik wdndO wdid;añl ,laIK we;sfõ' (𝟓 × 𝟑 = 𝟏𝟓)
(v) NO yd jdIamYS,S yhsfv%dldnk iïmQ¾Kfhkau mdfya jd;hg tla jkafka r:jdyk .ukd.ukh fya;=fjks'
fï ksid fudag¾ r: j,g W;afm%arl mßj¾;l iú lsÍfuka NO(g) , NO2 njg;a jdIamYS,S yhsfv%dldnk CO2
njg;a mßj¾;kh lr jdhqf.da,hg uqod yeÍu l< hq;=h' (10)
CHEMISTRY 14 Amila Dasanayake
You might also like
- Instructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYFrom EverandInstructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYNo ratings yet
- Plastic Films Made From Low-Density Polyethylene and Linear Low-Density Polyethylene For General Use and Packaging ApplicationsDocument5 pagesPlastic Films Made From Low-Density Polyethylene and Linear Low-Density Polyethylene For General Use and Packaging ApplicationsNasrin Akhondi100% (1)
- Answers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesFrom EverandAnswers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesRating: 1.5 out of 5 stars1.5/5 (2)
- คณิตศาสตร์Document34 pagesคณิตศาสตร์Atcharaporn UparaNo ratings yet
- Analytic Geometry: Graphic Solutions Using Matlab LanguageFrom EverandAnalytic Geometry: Graphic Solutions Using Matlab LanguageNo ratings yet
- Bentonite Slurry and Its UsesDocument4 pagesBentonite Slurry and Its UsesMajor Tushar100% (3)
- Crude CalculationDocument7 pagesCrude CalculationReetam Bose75% (4)
- Pamphlet 121 - Explosive Properties - Ed. 3 - 01-2009Document37 pagesPamphlet 121 - Explosive Properties - Ed. 3 - 01-2009sangoi87No ratings yet
- Lesson Plan On PercentageDocument9 pagesLesson Plan On PercentageBryanJAbuloc100% (2)
- Reduction of CamphorDocument8 pagesReduction of CamphorKevin Chen100% (4)
- MJC JC2 H2 Maths 2012 Year End Exam Paper 2 Solutions PDFDocument10 pagesMJC JC2 H2 Maths 2012 Year End Exam Paper 2 Solutions PDFRyan LeeNo ratings yet
- PT-06 - A SolDocument7 pagesPT-06 - A SolDanraj MeenaNo ratings yet
- Math 2014 June MemoDocument7 pagesMath 2014 June Memokarabelobelo2No ratings yet
- Lecture8 Saadat REVDocument29 pagesLecture8 Saadat REVJm NeutronNo ratings yet
- Solomon A MSDocument4 pagesSolomon A MSgranadeclamoNo ratings yet
- Companion-Soln bk5Document6 pagesCompanion-Soln bk5samthegofNo ratings yet
- Worksheet Quadratic Equations PDFDocument6 pagesWorksheet Quadratic Equations PDFLudovicaNo ratings yet
- Practice 3 SolutDocument4 pagesPractice 3 Solutyandi99No ratings yet
- Analisis Data Unit 1-1Document4 pagesAnalisis Data Unit 1-1Reni AstutiNo ratings yet
- Paper 3 Structure Essay MarkingDocument4 pagesPaper 3 Structure Essay MarkingNavodi SenevirathneNo ratings yet
- BDA31103 - LECT03 - 2DOF - Part 2 - Sem 2 1617Document30 pagesBDA31103 - LECT03 - 2DOF - Part 2 - Sem 2 1617Ahmad FikriNo ratings yet
- Test-2 SolutionsDocument11 pagesTest-2 SolutionspreethiNo ratings yet
- 2004 - Chimie - Internationala - Solutii - Clasa A XII-a - 0 PDFDocument20 pages2004 - Chimie - Internationala - Solutii - Clasa A XII-a - 0 PDFiugulescu laurentiuNo ratings yet
- Kunci Jawaban Fisika Klas X Bab 1 Martin Kanginan1Document37 pagesKunci Jawaban Fisika Klas X Bab 1 Martin Kanginan1Yogastio Esadimmarca100% (4)
- Asst 3Document10 pagesAsst 3nn1129374No ratings yet
- LT Silvio RevisiDocument15 pagesLT Silvio Revisisilvio andiNo ratings yet
- Bab 1Document37 pagesBab 1Ben Yudha SatriaNo ratings yet
- A A M WMC C 1) A WMC WMC A M Ma Mca Ma Mca Ma Ma Mca Mca Ma A WMC M + ( 2 M + MC WMC) A MC+ Ma) A M MC) A MC 2 M WMC) A MDocument4 pagesA A M WMC C 1) A WMC WMC A M Ma Mca Ma Mca Ma Ma Mca Mca Ma A WMC M + ( 2 M + MC WMC) A MC+ Ma) A M MC) A MC 2 M WMC) A Mtahirtp02No ratings yet
- Exam3 SolutionsDocument5 pagesExam3 SolutionsOrlando FernandezNo ratings yet
- 12-11-2023 SR - Elite & Target (C-120, C-Ipl & Ipl-Ic) - Jee Main - ctm-06 - Key & Sol'sDocument16 pages12-11-2023 SR - Elite & Target (C-120, C-Ipl & Ipl-Ic) - Jee Main - ctm-06 - Key & Sol'skishanchandan555No ratings yet
- Definite IntegralDocument3 pagesDefinite IntegraljillzoNo ratings yet
- Dokumen - Tips Homework 3 Solution Department of Statistics Ovitekstat526 Spring11filespdfshw3 SolpdfstatDocument12 pagesDokumen - Tips Homework 3 Solution Department of Statistics Ovitekstat526 Spring11filespdfshw3 Solpdfstatbourday RachidNo ratings yet
- Calculation - CP Deltah DeltagibbsDocument6 pagesCalculation - CP Deltah DeltagibbsYasemin KaradağNo ratings yet
- JP XII Physical&Inorganic Chemistry (19) - Prev Chaps - Inorg. Chem-1Document13 pagesJP XII Physical&Inorganic Chemistry (19) - Prev Chaps - Inorg. Chem-1Nibha PandeyNo ratings yet
- O-Levels Mathematics 2006 AnswersDocument13 pagesO-Levels Mathematics 2006 AnswersKuziva MbeuNo ratings yet
- Continuum Mechanics and PlasticityDocument2 pagesContinuum Mechanics and PlasticityWilson NaranjoNo ratings yet
- 10.1201 9780203491997-7 PDFDocument2 pages10.1201 9780203491997-7 PDFWilson NaranjoNo ratings yet
- 10.1201 9780203491997-7 PDFDocument2 pages10.1201 9780203491997-7 PDFWilson NaranjoNo ratings yet
- 4 < − 1 2 ≤ ≤ θ θ θ θ θ θ (2sin) 4 (2 sin) 2 1 (2 sin) 4 − + + ≥ + 2sin 4 < − 2si 2 1 n ≤ ≤ θDocument6 pages4 < − 1 2 ≤ ≤ θ θ θ θ θ θ (2sin) 4 (2 sin) 2 1 (2 sin) 4 − + + ≥ + 2sin 4 < − 2si 2 1 n ≤ ≤ θNg Shu QingNo ratings yet
- HwsDocument9 pagesHwshudha69No ratings yet
- UT FSRG2 Test-3 Code-A2 SolDocument4 pagesUT FSRG2 Test-3 Code-A2 SolKrishna NayakNo ratings yet
- Tugas 2 MTKDocument4 pagesTugas 2 MTKAsri SulastriNo ratings yet
- Algebra 1 A 4Document23 pagesAlgebra 1 A 4bryanNo ratings yet
- QB 2a03 Identities-ExtractedDocument24 pagesQB 2a03 Identities-ExtractedJackson WongNo ratings yet
- Ejercicios Potencias 4º AplcadasDocument2 pagesEjercicios Potencias 4º AplcadasantonioNo ratings yet
- Problem Solutions For Chapter 3Document12 pagesProblem Solutions For Chapter 3api-19870706No ratings yet
- 1 Modul Penggunaan MS Excel Untuk Perhitungan Teknik (Dasar1)Document5 pages1 Modul Penggunaan MS Excel Untuk Perhitungan Teknik (Dasar1)vincenvincen26No ratings yet
- 2020AL Chemistry MCQ - Amila DasanayakeDocument13 pages2020AL Chemistry MCQ - Amila Dasanayakeyopilo4090No ratings yet
- MESINDocument3 pagesMESINFierryal NafilaNo ratings yet
- (@bohring - Bot) ANSWER KEYDocument10 pages(@bohring - Bot) ANSWER KEYPratham MittalNo ratings yet
- Examples On Laplace TransformDocument2 pagesExamples On Laplace TransformMehmet AKBABANo ratings yet
- Cape 2015 Pure Math - Unit 1 P 02 SolutionsDocument5 pagesCape 2015 Pure Math - Unit 1 P 02 Solutionskayla morrisonNo ratings yet
- Lathi Ls SM Chapter 2Document33 pagesLathi Ls SM Chapter 2Dr Satyabrata JitNo ratings yet
- Probability and Statistic (SET1)Document9 pagesProbability and Statistic (SET1)Syamimi AtirahNo ratings yet
- Local Media3063370822841076474Document11 pagesLocal Media3063370822841076474Jade Albert G. FalladoNo ratings yet
- 2 Chemistry - Chemical Kinetics - 2 60 SolutionsDocument10 pages2 Chemistry - Chemical Kinetics - 2 60 SolutionsPRUTHVINo ratings yet
- Chapter 02Document82 pagesChapter 02jesus bastardoNo ratings yet
- Assignment of CSE201Document24 pagesAssignment of CSE201saifhossain.meNo ratings yet
- Laplace Transforms Practice Sheet PDFDocument3 pagesLaplace Transforms Practice Sheet PDFBilly MulengaNo ratings yet
- Quiz 1 Answer KeyDocument4 pagesQuiz 1 Answer KeyJohn Lloyd ComiaNo ratings yet
- Chemical Kinetics Exercise SolutionsDocument98 pagesChemical Kinetics Exercise SolutionsKivilia EduventuresNo ratings yet
- Tapal Kuda & Circular - Raynold Frinata Makuku - 11.2014.1.00466Document4 pagesTapal Kuda & Circular - Raynold Frinata Makuku - 11.2014.1.00466Aprilia Dwi AstutiNo ratings yet
- HW 3 SolutionsDocument9 pagesHW 3 SolutionsnadunnnNo ratings yet
- Universidad Católica de Santa Maria Escuela Profesional de Ing Mecanica Mecanica Eléctrica Y MecatronicaDocument8 pagesUniversidad Católica de Santa Maria Escuela Profesional de Ing Mecanica Mecanica Eléctrica Y MecatronicaBryan SierraNo ratings yet
- Oxydur PTB Solution 1Document6 pagesOxydur PTB Solution 1KelvinNo ratings yet
- This Study Resource Was: Names: Barquilla, Frenz BrianDocument7 pagesThis Study Resource Was: Names: Barquilla, Frenz BrianOne OwnNo ratings yet
- MSA Global EU UK RoHS Declaration 09072022Document15 pagesMSA Global EU UK RoHS Declaration 09072022Felipe CarmonaNo ratings yet
- Che 0417 FR3 MPBDocument13 pagesChe 0417 FR3 MPBDario BonillaNo ratings yet
- LWT - Food Science and Technology: Mohammad Imtiyaj Khan, P.S.C. Sri Harsha, P. Giridhar, G.A. RavishankarDocument9 pagesLWT - Food Science and Technology: Mohammad Imtiyaj Khan, P.S.C. Sri Harsha, P. Giridhar, G.A. RavishankarJoulesNo ratings yet
- Analytical ProcessDocument15 pagesAnalytical ProcessJeth CahayaganNo ratings yet
- Copper-Catalyzed de Uorinative Borylation and Silylation of Gem-Di Uoroallyl GroupsDocument5 pagesCopper-Catalyzed de Uorinative Borylation and Silylation of Gem-Di Uoroallyl GroupsUrmi Bhusan BhaktaNo ratings yet
- Auxiblocker SBDocument1 pageAuxiblocker SBphasithNo ratings yet
- Carbonic AcidDocument6 pagesCarbonic AcidPierangelo CarozzaNo ratings yet
- Ballova Et AlDocument11 pagesBallova Et AlDian SetyaNo ratings yet
- Validation of Contact Plates For Environmental MonitoringDocument5 pagesValidation of Contact Plates For Environmental Monitoringrobit SiddikiNo ratings yet
- Electrocleaning Technical BriefDocument8 pagesElectrocleaning Technical BriefSutopoNo ratings yet
- 〈1160〉 PHARMACEUTICAL CALCULATIONS IN PHARMACY PRACTICEDocument27 pages〈1160〉 PHARMACEUTICAL CALCULATIONS IN PHARMACY PRACTICEAptkeluarga SastraNo ratings yet
- First: Contact KitDocument72 pagesFirst: Contact KitAlex MungaiNo ratings yet
- Language Chemistry: Synopsis - 1Document20 pagesLanguage Chemistry: Synopsis - 1Snigdharani SahooNo ratings yet
- Synthesizing, Characterizing, and Toxicity Evaluating of Phycocyanin-ZnO Nanorod Composites-A Back To Nature ApproachesDocument32 pagesSynthesizing, Characterizing, and Toxicity Evaluating of Phycocyanin-ZnO Nanorod Composites-A Back To Nature ApproachesHossein ShahbaniNo ratings yet
- Soil Mechanics Laboratory: (3) : Grain Size Analysis - (Sieve Method+ Hydrometer Method)Document5 pagesSoil Mechanics Laboratory: (3) : Grain Size Analysis - (Sieve Method+ Hydrometer Method)Rana Abdelbaset BostanjiNo ratings yet
- Quaker Color: Technical Data SheetDocument3 pagesQuaker Color: Technical Data SheetAPEX SONNo ratings yet
- GenChem1 Periodical Test 1Document3 pagesGenChem1 Periodical Test 1MA. HAZEL TEOLOGONo ratings yet
- APCS-20A HempelDocument10 pagesAPCS-20A HempelarjunmohananNo ratings yet
- Proposal Writing GuidelineDocument31 pagesProposal Writing GuidelinemasdfgNo ratings yet
- Properties of CementDocument6 pagesProperties of CementJa Phe TiNo ratings yet
- CM-9CB TG-S9Cb US-9CbDocument3 pagesCM-9CB TG-S9Cb US-9Cbamit singhNo ratings yet
- Paper and PulpDocument13 pagesPaper and PulpSanjaya NaralNo ratings yet