Professional Documents
Culture Documents
Alloying Elements
Uploaded by
Prabhat Tanti0 ratings0% found this document useful (0 votes)
0 views2 pagesfor material science
Original Title
Alloying elements
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentfor material science
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
0 views2 pagesAlloying Elements
Uploaded by
Prabhat Tantifor material science
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
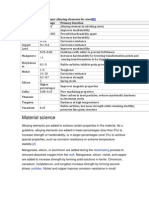
The properties, which may be improved
Stabilizing austenite – increasing the temperature range, in which austenite
exists.
The elements, having the same crystal structure as that of austenite (cubic face centered –
FCC), raise the A4 point (the temperature of formation of austenite from liquid phase) and
decrease the A3 temperature.
These elements are nickel (Ni), manganese (Mn), cobalt (Co) and copper (Cu).
Examples of austenitic steels: austenitic stainless steels, Hadfield steel (1%C, 13%Mn,
1.2%Cr).
Stabilizing ferrite – decreasing the temperature range, in which austenite exists.
The elements, having the same crystal structure as that of ferrite (cubic body centered –
BCC), lower the A4 point and increase the A3 temperature.
These elements lower the solubility of carbon in austenite, causing increase of amount of
carbides in the steel.
The following elements have ferrite stabilizing effect: chromium (Cr), tungsten (W),
Molybdenum (Mo), vanadium (V), aluminum (Al) and silicon (Si).
Examples of ferritic steels:transformer sheets steel (3%Si), F-Cr alloys.
Carbide forming – elements forming hard carbides in steels.
The elements like chromium (Cr), tungsten (W), molybdenum (Mo), vanadium (V), titanium
(Ti), niobium (Nb), tantalum (Ta), zirconium (Zr) form hard (often complex) carbides,
increasing steel hardness and strength.
Examples of steels containing relatively high concentration of carbides: hot work tool
steels, high speed steels.
Carbide forming elements also form nitrides reacting with Nitrogen in steels.
Graphitizing – decreasing stability of carbides, promoting their breaking and
formation of free Graphite.
The following elements have graphitizing effect: silicon (Si), nickel (Ni), cobalt (Co),
aluminum (Al).
Decrease of the eutectoid concentration.
The following elements lower eutectoid concentration of carbon: titanium (Ti), molybdenum
(Mo), tungsten (W), silicon (Si), chromium (Cr), nickel (Ni).
Increase of corrosion resistance.
Aluminum (Al), silicon (Si), and chromium (Cr) form thin an strong oxide film on the steel
surface, protecting it from chemical attacks.
Characteristics of alloying elements
Manganese (Mn) – improves hardenability, ductility and wear resistance. Mn eliminates
formation of harmful iron sulfides, increasing strength at high temperatures.
Nickel (Ni) – increases strength, impact strength and toughness, impart corrosion
resistance in combination with other elements.
Chromium (Cr) – improves hardenability, strength and wear resistance, sharply increases
corrosion resistance at high concentrations (> 12%).
Tungsten (W) – increases hardness particularly at elevated temperatures due to stable
carbides, refines grain size.
Vanadium (V) – increases strength, hardness, creep resistance and impact resistance due
to formation of hard vanadium carbides, limits grain size.
Molybdenum (Mo) – increases hardenability and strength particularly at high
temperatures and under dynamic conditions.
Silicon (Si) – improves strength, elasticity, acid resistance and promotes large grain sizes,
which cause increasing magnetic permeability.
Titanium (Ti) – improves strength and corrosion resistance, limits austenite grain size.
Cobalt (Co) – improves strength at high temperatures and magnetic permeability.
Zirconium (Zr) – increases strength and limits grain sizes.
Boron (B) – highly effective hardenability agent, improves deformability and machinability.
Copper (Cu) – improves corrosion resistance.
Aluminum (Al) – deoxidizer, limits austenite grains growth.
You might also like
- Hematology - Steininger ReviewDocument30 pagesHematology - Steininger ReviewIssa AlejoNo ratings yet
- Diagnosis Tools For Humidity Born Surface Contaminants On L - 2022 - Journal ofDocument7 pagesDiagnosis Tools For Humidity Born Surface Contaminants On L - 2022 - Journal ofmohammedNo ratings yet
- Effect of Alloying Elements On Steel PropertiesDocument2 pagesEffect of Alloying Elements On Steel PropertiesKARTHIGEYAN.RNo ratings yet
- Effect of alloying elements on steel propertiesDocument1 pageEffect of alloying elements on steel propertiesmishtinilNo ratings yet
- Effect of Alloying Elements On Steel Properties (SubsTech)Document2 pagesEffect of Alloying Elements On Steel Properties (SubsTech)hguptabhel100% (1)
- General Information About SteelsDocument20 pagesGeneral Information About SteelsilkinNo ratings yet
- Effects of Alloying Elements on Al Properties (39Document3 pagesEffects of Alloying Elements on Al Properties (39Rex RusselNo ratings yet
- Material & Technology: Alloy: An Alloy Is A Metal Composed of More Than One Element. EngineeringDocument4 pagesMaterial & Technology: Alloy: An Alloy Is A Metal Composed of More Than One Element. Engineeringapi-19753215No ratings yet
- Ferrous and non-Ferous metalsDocument21 pagesFerrous and non-Ferous metalsNicollas MatheusNo ratings yet
- Plain Carbon SteelDocument6 pagesPlain Carbon Steelحسين كاظم ياسينNo ratings yet
- Effect of Alloying Element in AluminiumDocument3 pagesEffect of Alloying Element in Aluminiumvikrant GarudNo ratings yet
- local_media FILEDocument7 pageslocal_media FILEmacksabado78No ratings yet
- Alloying Elements in SteelDocument5 pagesAlloying Elements in Steelkihal zohirNo ratings yet
- Engineering MaterialsDocument32 pagesEngineering MaterialsAdhanom G.No ratings yet
- Effect of Alloying Elements On Steel PropertiesDocument5 pagesEffect of Alloying Elements On Steel PropertiesgovimanoNo ratings yet
- Materials Science and Engineering-Chapter 11Document3 pagesMaterials Science and Engineering-Chapter 11JurgenNo ratings yet
- Steel TopicDocument6 pagesSteel Topicazher03No ratings yet
- Alloys SampleDocument10 pagesAlloys Sampley ki yudi rapperNo ratings yet
- 211 2aDocument33 pages211 2aMada ChohNo ratings yet
- Stamping 101: Material Guidelines: Properties and Characteristics That Affect FormabilityDocument5 pagesStamping 101: Material Guidelines: Properties and Characteristics That Affect FormabilityDavid RodriguezNo ratings yet
- Steels For Aerospace Engineering Jan2020.6310.1580092900.0839Document78 pagesSteels For Aerospace Engineering Jan2020.6310.1580092900.0839EudoNo ratings yet
- Applicarion of Alloy SteelsDocument4 pagesApplicarion of Alloy SteelsMersal VijayNo ratings yet
- Alloying Elements ExcelDocument18 pagesAlloying Elements ExcelRavindra ErabattiNo ratings yet
- Corrosioncontrol 150425135701 Conversion Gate01Document62 pagesCorrosioncontrol 150425135701 Conversion Gate01bibek paikNo ratings yet
- Steel ManufacturingDocument29 pagesSteel ManufacturingNDTInstructor100% (3)
- Ferrous Metals and AlloysDocument44 pagesFerrous Metals and AlloysLeonardDacaymatNo ratings yet
- Ch13 Materials ApplicationsDocument63 pagesCh13 Materials ApplicationsThefairman UnkownNo ratings yet
- Me17 6-9 PDFDocument55 pagesMe17 6-9 PDFMico CampoNo ratings yet
- Alloy SteelDocument9 pagesAlloy Steelamr_scorpion_engNo ratings yet
- MZ FS Unit - 1Document27 pagesMZ FS Unit - 1Jai KumarNo ratings yet
- Shipbuilding Materials and Metallurgy: Alloy Steels and Their PropertiesDocument41 pagesShipbuilding Materials and Metallurgy: Alloy Steels and Their PropertiesMahadi HasanNo ratings yet
- MEE5003 - MOD2 - LEC2 - SteelsDocument29 pagesMEE5003 - MOD2 - LEC2 - SteelsAbid YusufNo ratings yet
- Unit Iii: V 6Cwodc-3VrkDocument237 pagesUnit Iii: V 6Cwodc-3VrkDinesh KumarNo ratings yet
- Material Science: Prof. Satish V. KailasDocument12 pagesMaterial Science: Prof. Satish V. KailasAlvin SmithNo ratings yet
- Piping MaterialDocument45 pagesPiping MaterialLcm TnlNo ratings yet
- C-MN SteelsDocument48 pagesC-MN SteelsEri Dya FadliNo ratings yet
- Ch-27.7 Alloying Element of Steel and Alloy SteelDocument51 pagesCh-27.7 Alloying Element of Steel and Alloy SteelSmruti Ranjan PattanayakNo ratings yet
- Alloy SteelDocument5 pagesAlloy SteelKun Hadipati Kusuma NegaraNo ratings yet
- Bme - Part 1Document49 pagesBme - Part 1Sumanth ChallaNo ratings yet
- Tratamientos Térmicos: Carlos Bohórquez 2010Document61 pagesTratamientos Térmicos: Carlos Bohórquez 2010procesosun2010No ratings yet
- Chapter 2 Alloys - 2012 - Applied Welding EngineeringDocument5 pagesChapter 2 Alloys - 2012 - Applied Welding EngineeringJames LeonNo ratings yet
- Different Types of Steel Grades and Their PropertiesDocument9 pagesDifferent Types of Steel Grades and Their PropertiesSyed Shoaib RazaNo ratings yet
- Ch-27.7 Alloying Element of Steel and Alloy SteelDocument50 pagesCh-27.7 Alloying Element of Steel and Alloy SteelPARESHNo ratings yet
- Lecture 9 - Plain Carbon Steels - 2013Document45 pagesLecture 9 - Plain Carbon Steels - 2013ArunNo ratings yet
- G H Patel College of Engineering and TechnologyDocument28 pagesG H Patel College of Engineering and TechnologyAnonymous XwaOunGKNo ratings yet
- SANDVIK What Is Stainless SteelDocument7 pagesSANDVIK What Is Stainless Steelpipedown456No ratings yet
- Engineering Materials: Metals and Their Alloys Ceramics Polymers CompositesDocument53 pagesEngineering Materials: Metals and Their Alloys Ceramics Polymers CompositesSyed Muhammad AliNo ratings yet
- Chromium:-: 21 Chemical Elements and Effects On Steel Mechanical PropertiesDocument8 pagesChromium:-: 21 Chemical Elements and Effects On Steel Mechanical Propertiesdhoni03No ratings yet
- Werkstoffkunde Der Stähle - Kurzzusammenfassung en-US - UnlockedDocument8 pagesWerkstoffkunde Der Stähle - Kurzzusammenfassung en-US - UnlockedLorena juárezNo ratings yet
- Ferrous and Non-Ferrous Alloys GuideDocument22 pagesFerrous and Non-Ferrous Alloys GuideHarsh V Ashok0% (1)
- Alloying Metal Effect in SteelDocument1 pageAlloying Metal Effect in SteelakankshaNo ratings yet
- Alloy SteelsDocument2 pagesAlloy Steelswhi7efea7herNo ratings yet
- AlloysDocument91 pagesAlloysNiccoloNo ratings yet
- Alloying Elements of Steels and PropertiesDocument3 pagesAlloying Elements of Steels and PropertiesdaimaheshNo ratings yet
- FC-06-Engineering Material & Metallurgy PDFDocument431 pagesFC-06-Engineering Material & Metallurgy PDFsomnath ghosh100% (1)
- Anirudha Samant REG NO.-16BME1044 Slot - C1 Alloy SteelsDocument12 pagesAnirudha Samant REG NO.-16BME1044 Slot - C1 Alloy SteelsAnirudhaNo ratings yet
- Manufacturing Materials ColouredDocument20 pagesManufacturing Materials Colouredramu reddyNo ratings yet
- Manuf. Tech. - IntroductionDocument38 pagesManuf. Tech. - IntroductionManuel Tikongyin WundengbaNo ratings yet
- ALLOYS: PROPERTIES AND USESDocument9 pagesALLOYS: PROPERTIES AND USESMadhavanIceNo ratings yet
- Ferrous Alloys GuideDocument56 pagesFerrous Alloys Guidejayakrishnan psNo ratings yet
- Die Casting Metallurgy: Butterworths Monographs in MaterialsFrom EverandDie Casting Metallurgy: Butterworths Monographs in MaterialsRating: 3.5 out of 5 stars3.5/5 (2)
- KhuDocument2 pagesKhuPrabhat TantiNo ratings yet
- Steel Numbering PDFDocument29 pagesSteel Numbering PDFAKSHAY BHATKARNo ratings yet
- Stainless steel properties and applicationsDocument2 pagesStainless steel properties and applicationsPrabhat TantiNo ratings yet
- Composite MaterialsDocument30 pagesComposite MaterialsPrabhat TantiNo ratings yet
- YyyDocument1 pageYyyPrabhat TantiNo ratings yet
- GhiDocument2 pagesGhiPrabhat TantiNo ratings yet
- PeDocument2 pagesPePrabhat TantiNo ratings yet
- Root LocusDocument2 pagesRoot LocusPrabhat TantiNo ratings yet
- PokDocument2 pagesPokPrabhat TantiNo ratings yet
- EM Manual-1Document34 pagesEM Manual-1sahebraoNo ratings yet
- GHTDocument3 pagesGHTPrabhat TantiNo ratings yet
- AsdDocument4 pagesAsdPrabhat TantiNo ratings yet
- Practical Questions AnsDocument2 pagesPractical Questions AnsPrabhat TantiNo ratings yet
- Reasoning 1Document3 pagesReasoning 1Prabhat TantiNo ratings yet
- NIT Durgapur ShortlistDocument6 pagesNIT Durgapur ShortlistPrabhat TantiNo ratings yet
- Parallel Operation PDFDocument4 pagesParallel Operation PDFjitendra meenaNo ratings yet
- Synchronous MachinesDocument31 pagesSynchronous Machinesrambala123No ratings yet
- Prof. Ch. SAI BABU: TopicDocument90 pagesProf. Ch. SAI BABU: TopicAbhimanyu Perumal100% (3)
- AnnalPrg Exam RT 2022 Engl 130821Document1 pageAnnalPrg Exam RT 2022 Engl 130821Pruthaviraj BNo ratings yet
- Chem 2206 Unit 3Document56 pagesChem 2206 Unit 3Danica Rose ZapanzaNo ratings yet
- Biology - Physics Chemistry MCQS: Gyanm'S General Awareness - November 2014Document13 pagesBiology - Physics Chemistry MCQS: Gyanm'S General Awareness - November 2014santosh.manojNo ratings yet
- Sri Chaitanya IIT Academy., India.: 2015 - PAPER-IIDocument17 pagesSri Chaitanya IIT Academy., India.: 2015 - PAPER-IISamarth ThakurNo ratings yet
- Pi Is 0021925819649951Document6 pagesPi Is 0021925819649951Abas NjarkhatirNo ratings yet
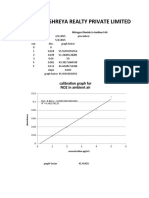
- Shreya Realty Private Limited: Calibration Graph For NO2 in Ambient AirDocument7 pagesShreya Realty Private Limited: Calibration Graph For NO2 in Ambient AirKushal SharmaNo ratings yet
- Acid, Base and Salt NoteDocument40 pagesAcid, Base and Salt NoteSamuel WilliamsNo ratings yet
- CL 514: Fundamentals of Material Science & Engineering Crystal Structures and PropertiesDocument25 pagesCL 514: Fundamentals of Material Science & Engineering Crystal Structures and PropertiesABHISHEK KUMARNo ratings yet
- 1045 Exp5 ObservingclassifyingreactionsDocument18 pages1045 Exp5 ObservingclassifyingreactionsPeluzitaNo ratings yet
- Standard Methods For The Examination of Water and Wastewater 23th (Rodger B. Baird, Eugene W. Rice Etc.) (Z-Library) - 1-2Document2 pagesStandard Methods For The Examination of Water and Wastewater 23th (Rodger B. Baird, Eugene W. Rice Etc.) (Z-Library) - 1-2almirantebenbowNo ratings yet
- Multilink Hybrid Abutment CementDocument40 pagesMultilink Hybrid Abutment CementKJ ChaeNo ratings yet
- Eastman - Dimethylformamide (DMF)Document4 pagesEastman - Dimethylformamide (DMF)Darshan PatelNo ratings yet
- A Review of Top-Down and Bottom-Up Synthesis Methods For The Production of Graphene, Graphene Oxide and Reduced Graphene OxideDocument36 pagesA Review of Top-Down and Bottom-Up Synthesis Methods For The Production of Graphene, Graphene Oxide and Reduced Graphene OxideRodrigo Gabriel BuenoNo ratings yet
- Water quality analysis methods and index calculationDocument1 pageWater quality analysis methods and index calculationImad Habeeb ObeadNo ratings yet
- Group 18 elements and the periodic tableDocument1 pageGroup 18 elements and the periodic tableDhara PandeyNo ratings yet
- PHG283-chap2 - Pharmacological Screening Programs For Plant Extracts - Part 1Document117 pagesPHG283-chap2 - Pharmacological Screening Programs For Plant Extracts - Part 1Gaurav AdhikariNo ratings yet
- Polymers - Controlled Drug Delivery SystemsDocument43 pagesPolymers - Controlled Drug Delivery Systemsmonika1983007No ratings yet
- Chemistry - Ch-1 NotesDocument5 pagesChemistry - Ch-1 NoteskomalNo ratings yet
- Biology and Diversity of Viruses, Bacteria and Fungi (Paper Code: Bot 501)Document25 pagesBiology and Diversity of Viruses, Bacteria and Fungi (Paper Code: Bot 501)Ajzm CompanyNo ratings yet
- Practical 1BDocument8 pagesPractical 1BHoe Lam WanNo ratings yet
- DOWSIL™ 2-9034 Emulsion: Features & BenefitsDocument5 pagesDOWSIL™ 2-9034 Emulsion: Features & BenefitsLaban KantorNo ratings yet
- Fluid Thioglycollate Medium (Thioglycollate Medium W/ Indicator and Dextrose)Document1 pageFluid Thioglycollate Medium (Thioglycollate Medium W/ Indicator and Dextrose)sinaNo ratings yet
- VS TL - Urine - L1 28564 2112 2Document24 pagesVS TL - Urine - L1 28564 2112 2mnemonicsNo ratings yet
- Continuous Flow Bioreactor: Manam Walait Lecturer FLS, Ucp LahoreDocument13 pagesContinuous Flow Bioreactor: Manam Walait Lecturer FLS, Ucp LahoreJawadNo ratings yet
- Construction and Building Materials: Hrishikesh A Shahane, Satyajit PatelDocument12 pagesConstruction and Building Materials: Hrishikesh A Shahane, Satyajit PatelBustan ShahNo ratings yet
- Namma Kalvi 11th Chemistry Unit 11 Study Material em 215587Document9 pagesNamma Kalvi 11th Chemistry Unit 11 Study Material em 215587prathiksha6660No ratings yet
- EUROPHARM IDENTIFICATION REACTIONSDocument4 pagesEUROPHARM IDENTIFICATION REACTIONSRaficaNo ratings yet
- Introduction To NanomaterialsDocument73 pagesIntroduction To Nanomaterialsabhay yelmuleNo ratings yet