Professional Documents
Culture Documents
TD 1 Properties Enonce
Uploaded by
Luc AusterCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
TD 1 Properties Enonce
Uploaded by
Luc AusterCopyright:
Available Formats
1. TP (properties).pdf.
Gabrielgarcy12
Ingeniería Térmica
2º Grado en Ingeniería Mecánica
Escuela Politécnica Superior
Universidad Carlos III de Madrid
Reservados todos los derechos.
No se permite la explotación económica ni la transformación de esta obra. Queda permitida la impresión en su totalidad.
No se permite la explotación económica ni la transformación de esta obra. Queda permitida la impresión en su totalidad.
PROPERTIES
1.1.Evaluate the following water properties.
P (kPa) T (ºC) x (%) v (m3/kg) u (kJ/kg) h (kJ/kg) s (kJ/kgK)
90000 150
600 1
• Application example: evaluating properties in two-phase regions (saturation
state).
P (kPa) T (ºC) x (%) v (m3/kg) u (kJ/kg) h (kJ/kg) s (kJ/kgK)
5 70
Reservados todos los derechos.
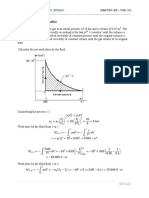
1.2. A closed, rigid container of 0.17 m3 initially holds water vapor at p1 = 500 kPa and
T1 = 275 ºC. The temperature drops as a result of heat transfer to the surroundings until
its temperature is 90 ºC. Plot the process on a P-v and a T-v diagram. What is the vapor
mass contained in the deposit at the final state? What is the pressure? At which
temperature will the phase change start? Sol: 0.07 kg, 75 kPa, 140 ºC
1.3. A mass of 2 kg of water initially at P = 20 MPa and T = 700 °C undergoes the
following processes. Draw roughly each process and calculate the variations of the
internal energy and entropy of the mass of water in each process.
a) Isentropic process until reaching T =125 ºC
b) Isochoric process until reaching T =125 ºC
c) Isobaric process until reaching T =125 ºC
d) Isenthalpic process until reaching P = 5 bar
e) Isothermal process until reaching P = 5 bar
Re: a) -1.9 MJ, 0 J/K; b) -5.6 MJ, -10.2 kJ/K; c*) -5.7 MJ, -10.6 kJ/K; d) -110 kJ, 3.3
kJ/K; e) 180 kJ, 3.6 kJ/K * Using ideal liquid model for the final state
SUBSTANCE MODELS
2.1. 0.5 kg of air initially at 100kPa, 300K is heated at constant volume until the
temperature is 500 K. Assuming cp = 1.005kJ/(kg·K) and R = 0.287 kJ/(kg·K) constant
during the process (ideal gas model with constant specific heats), determine the final
pressure and the specific internal energy and entropy change of the system.
Sol: 167kPa; ΔU=71.80kJ; ΔS= 183J/K
• Isentropic process for ideal gas and incompressible liquids. Application
examples.
2.2. An ideal gas with constant cp = 1245 J/(kg·K) and cv = 830 J/(kg·K) undergoes an
isentropic expansion from p1 = 600 kPa and T1 = 1100 K to P2 = 100 kPa. Determine the
final temperature. Sol: 605K
2.3 Plot in P-v, T-v, T-s y P-h diagrams the following process: the compression of 1kg of
saturated liquid water from a pressure of 1 bar to the pressure of the critical point (22.09
MPa). The process is assumed isentropic. Determine the temperature, internal energy and
enthalpy change. Consider an incompressible liquid. Sol: 0K, 0J, 21.99kJ
a64b0469ff35958ef4ab887a898bd50bdfbbe91a-2100044
You might also like
- Fluid Mechanics Cengel (Solutions Manual) Chap12-001Document34 pagesFluid Mechanics Cengel (Solutions Manual) Chap12-001NURUL SYUHADA BT ISMAIL HAJAR50% (2)
- Module 7: Solved ProblemsDocument15 pagesModule 7: Solved Problemscaptainhass67% (6)
- Section-1: Material-Independent Property Relations: AnswersDocument20 pagesSection-1: Material-Independent Property Relations: Answersnkosana2No ratings yet
- RTV Silicone Rubber CoatingDocument7 pagesRTV Silicone Rubber CoatingJane ChatsiriphatthanaNo ratings yet
- Shell and Tube Heat Exchanger Sample Problem and SolutionDocument9 pagesShell and Tube Heat Exchanger Sample Problem and Solutionlouisealfonzo.chanNo ratings yet
- Sample - Solution Manual For Principles of Chemical Engineering Processes 1st Edition - Nayef Ghasem, Redhouane HendaDocument4 pagesSample - Solution Manual For Principles of Chemical Engineering Processes 1st Edition - Nayef Ghasem, Redhouane Hendaفراس الوافيNo ratings yet
- Thermo EXAMPLE 7.1-CHAPTER 7 PDFDocument11 pagesThermo EXAMPLE 7.1-CHAPTER 7 PDFFattihiEkhmalNo ratings yet
- CHME 5101, Fall 2021: Homework Assignment 3 (Due 10/13/21, 11:59 PM US Eastern Time) Problem 1Document4 pagesCHME 5101, Fall 2021: Homework Assignment 3 (Due 10/13/21, 11:59 PM US Eastern Time) Problem 1TosinNo ratings yet
- Question 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT DsDocument6 pagesQuestion 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT Dsfivos_rgNo ratings yet
- Assumptions 1 Steady Operating Conditions Exist. 2 The Mixing Chamber Is Well-Insulated So That Heat LossDocument1 pageAssumptions 1 Steady Operating Conditions Exist. 2 The Mixing Chamber Is Well-Insulated So That Heat LossHafiz Mahar28No ratings yet
- CCTD101B Tutorial 7 - EntropyDocument1 pageCCTD101B Tutorial 7 - EntropykibweantNo ratings yet
- Processes of Fluids: Theories Values & DisciplineDocument1 pageProcesses of Fluids: Theories Values & DisciplineLester SamsonNo ratings yet
- Question 1. During An Experiment Conducted in A Room at 25Document11 pagesQuestion 1. During An Experiment Conducted in A Room at 25fivos_rgNo ratings yet
- Adamson UniversityDocument3 pagesAdamson UniversityVanessa Elaine CaoNo ratings yet
- INME 4045 - Chapter 3 - Suggested ProblemsDocument2 pagesINME 4045 - Chapter 3 - Suggested ProblemsAmanda SotoNo ratings yet
- 2W04 Assignment 5 - 2023 Solutions PDFDocument4 pages2W04 Assignment 5 - 2023 Solutions PDFas asNo ratings yet
- 2023 MteDocument6 pages2023 MteISHAAN JAIN 22114039No ratings yet
- 1 Sessional Test (Even Semester)Document1 page1 Sessional Test (Even Semester)anadinath sharmaNo ratings yet
- Ke PeDocument11 pagesKe PeAriel Carlos De LeonNo ratings yet
- شيت واجبDocument3 pagesشيت واجبhussamjamal432No ratings yet
- KJ/KG 1 - 2988 C 0 35 Mpa 8 ¿ QDocument5 pagesKJ/KG 1 - 2988 C 0 35 Mpa 8 ¿ QKimi HakimiNo ratings yet
- Final Exam Thermodynamics QDocument4 pagesFinal Exam Thermodynamics QEnrico BorjaNo ratings yet
- Tutorial 7 (Lecture 7, Chapter 7) Engineering Mechanics DepartmentDocument3 pagesTutorial 7 (Lecture 7, Chapter 7) Engineering Mechanics DepartmentAhmedSeragNo ratings yet
- Thermo EXAMPLE 7.2-CHAPTER 7 PDFDocument33 pagesThermo EXAMPLE 7.2-CHAPTER 7 PDFFattihiEkhmalNo ratings yet
- TD WorksheetDocument4 pagesTD WorksheetrtyiookNo ratings yet
- EXAMDocument1 pageEXAMkelly evangelistaNo ratings yet
- Ctdy Tutorial 2Document3 pagesCtdy Tutorial 2Bright ChabweraNo ratings yet
- TDCE Question Bank - 2018 Unit IDocument11 pagesTDCE Question Bank - 2018 Unit IvinodNo ratings yet
- EXAM - (M) 2018: Mechanical Engineering Paper - IIDocument12 pagesEXAM - (M) 2018: Mechanical Engineering Paper - IISandeep PrajapatiNo ratings yet
- 1 FormatsDocument1 page1 FormatsmsloveindiaNo ratings yet
- Thermodynamics: Practice Problems For Property EvaluationDocument9 pagesThermodynamics: Practice Problems For Property EvaluationSaurav kumar silNo ratings yet
- Engg ThermodynamicsgfDocument3 pagesEngg Thermodynamicsgfphysics a2No ratings yet
- Tarea No 7 ExergiaDocument3 pagesTarea No 7 ExergiaAndres RomeroNo ratings yet
- 15me03 Thermodynamics Problems June2017Document19 pages15me03 Thermodynamics Problems June2017Praveen Vijay100% (1)
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Rankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S XDocument16 pagesRankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S Xanon_166336005No ratings yet
- Chapter 3 - ContentsDocument8 pagesChapter 3 - ContentsSaiful MunirNo ratings yet
- Sample - Solution Manual For Principles of Chemical Engineering Processes 1st Edition - Nayef Ghasem, Redhouane HendaDocument4 pagesSample - Solution Manual For Principles of Chemical Engineering Processes 1st Edition - Nayef Ghasem, Redhouane Hendaفراس الوافيNo ratings yet
- 2300 HW 11 Sol PDFDocument4 pages2300 HW 11 Sol PDFJose Luis Huaman AlbornozNo ratings yet
- Thermodynamics Derived FormulaDocument4 pagesThermodynamics Derived FormulaAlex AndersNo ratings yet
- Tutorial - 6 - EntropyDocument7 pagesTutorial - 6 - EntropyanotherdeobiNo ratings yet
- Chapter 1 - ExerciseDocument15 pagesChapter 1 - Exerciseحسن كميت hassankomeit lNo ratings yet
- 2300 HW 13 SolDocument4 pages2300 HW 13 SolFrederick DugayNo ratings yet
- Basics of ThermodynamicsDocument36 pagesBasics of ThermodynamicsYeditha Satyanarayana MurthyNo ratings yet
- Tarea 5 TermodinamicaDocument4 pagesTarea 5 TermodinamicaMario GonzalezNo ratings yet
- Final Exam 20172018 Sem 2Document10 pagesFinal Exam 20172018 Sem 2Abdulrahman DesoukyNo ratings yet
- Thermodynamic 2Document3 pagesThermodynamic 2LYRICALLY MEMEDNo ratings yet
- Thermo 1Document2 pagesThermo 1Diwas GhimireNo ratings yet
- TS 1 To 6Document11 pagesTS 1 To 6Anshul Gautam100% (1)
- MEG 212 Practise QuestionsdocxDocument11 pagesMEG 212 Practise Questionsdocxoyetunde ridwanNo ratings yet
- SKMM 2413 - Test 1 - 20172018 - Sem 1Document5 pagesSKMM 2413 - Test 1 - 20172018 - Sem 1Abdulrahman DesoukyNo ratings yet
- Phychem 1 Review 1 Sept 2015Document2 pagesPhychem 1 Review 1 Sept 2015Jupert Jasser AbellanaNo ratings yet
- Solutions ProblemSet8 Sem22007Document7 pagesSolutions ProblemSet8 Sem22007clearcastingNo ratings yet
- ThermodynamicsDocument1 pageThermodynamicsCharina RonquilloNo ratings yet
- Test 1 With AnsDocument4 pagesTest 1 With AnsKavinesh GanesanNo ratings yet
- ETD - Question BankDocument6 pagesETD - Question BankGopinath VNo ratings yet
- Final ExamDocument5 pagesFinal ExamWaqasNo ratings yet
- CHE Assignment 2Document2 pagesCHE Assignment 2Adesite GodwinNo ratings yet
- Fluid 9ed Solution Manual PDFDocument919 pagesFluid 9ed Solution Manual PDFDermz GayosoNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- 2021-2022 Mecanismo Ordinario EnonceDocument2 pages2021-2022 Mecanismo Ordinario EnonceLuc AusterNo ratings yet
- SFC Exercises EnonceDocument12 pagesSFC Exercises EnonceLuc Auster0% (1)
- Topic2 Part II Exercisessolutions Balance Sheet 2q2013 14 EnonceDocument13 pagesTopic2 Part II Exercisessolutions Balance Sheet 2q2013 14 EnonceLuc AusterNo ratings yet
- Ch5-Capacitance and DielectricsDocument46 pagesCh5-Capacitance and Dielectricsmehdii.heidary1366100% (3)
- EPEF 03-04 Problems Problems wk06 CorrDocument3 pagesEPEF 03-04 Problems Problems wk06 CorrLuc AusterNo ratings yet
- EPEF 03-04 Problems Problems wk06 CorrDocument3 pagesEPEF 03-04 Problems Problems wk06 CorrLuc AusterNo ratings yet
- Cambridge International Examinations Cambridge International General Certificate of Secondary EducationDocument20 pagesCambridge International Examinations Cambridge International General Certificate of Secondary EducationequakeroatsNo ratings yet
- Materials Science at The Nanoscale: C.N.R. RaoDocument12 pagesMaterials Science at The Nanoscale: C.N.R. RaoPratheeshVnNo ratings yet
- Science and Health 6Document2 pagesScience and Health 6MA. PATRIA MANDAPNo ratings yet
- I. Melc.: Perpetual Succour Academy, Inc. National Rd. Poblacion Dos, Malabuyoc, Cebu Science 10Document4 pagesI. Melc.: Perpetual Succour Academy, Inc. National Rd. Poblacion Dos, Malabuyoc, Cebu Science 10Cry BeroNo ratings yet
- Projet RockyDocument19 pagesProjet RockyCassella AdrianoNo ratings yet
- Indo Kiman 2Document8 pagesIndo Kiman 2HasdiNo ratings yet
- Orange Research Catalog SmallDocument32 pagesOrange Research Catalog SmallLuz Stella Calixto GomezNo ratings yet
- Design of SlabDocument7 pagesDesign of SlabAlam Mohammad Parvez SaifiNo ratings yet
- Chemical Bonding Project 1Document2 pagesChemical Bonding Project 1Queen ErellaNo ratings yet
- BlueSolar Grid Inverter 4 - ENDocument1 pageBlueSolar Grid Inverter 4 - ENAnthony MainaNo ratings yet
- DC 70D ManualDocument17 pagesDC 70D ManualAnonymous vqsuRyNo ratings yet
- Pset 2Document13 pagesPset 2rishiko aquinoNo ratings yet
- Type 900 Series Electrode Stabilization Ovens: Operating InstructionsDocument6 pagesType 900 Series Electrode Stabilization Ovens: Operating InstructionsSATHISH KUMARNo ratings yet
- SUper Perc-ElexDocument23 pagesSUper Perc-ElexNo MarNo ratings yet
- Exercises - Mag Circuits+ XFMRsDocument5 pagesExercises - Mag Circuits+ XFMRsMINH Nguyễn TuấnNo ratings yet
- User Manual: Product Type: Switching Power Supply Model Name: HG2, HP2, PSM, PSLDocument17 pagesUser Manual: Product Type: Switching Power Supply Model Name: HG2, HP2, PSM, PSLotrupon melliNo ratings yet
- Steel Beam BracketDocument10 pagesSteel Beam BracketMohammed AyeshNo ratings yet
- Single Core Unarmoured LV CablesDocument1 pageSingle Core Unarmoured LV CablesPrashanth ShastryNo ratings yet
- Iris Power RIV Camera System: Rotor-in-Place Visual Inspection of Rotating MachinesDocument2 pagesIris Power RIV Camera System: Rotor-in-Place Visual Inspection of Rotating MachinesamirNo ratings yet
- Kami Export - ALEXA CADENA - PhotosynthesisLabSE - Google Doc VersionDocument5 pagesKami Export - ALEXA CADENA - PhotosynthesisLabSE - Google Doc VersionALEXA CADENANo ratings yet
- Chapter 11: Magnetic Materials: EXERCISES (PAGES 263 - 264)Document14 pagesChapter 11: Magnetic Materials: EXERCISES (PAGES 263 - 264)sagarshivnathsingh18818No ratings yet
- Greene Tweed Arlon 1000 Typical Property SheetDocument3 pagesGreene Tweed Arlon 1000 Typical Property SheetYang WangNo ratings yet
- Project ReportDocument14 pagesProject ReportIsmail ShekhNo ratings yet
- Fiitjee: Sample PaperDocument19 pagesFiitjee: Sample PaperApoorv DarshanNo ratings yet
- Siemens Power Engineering Guide 7E 343Document1 pageSiemens Power Engineering Guide 7E 343mydearteacherNo ratings yet
- AC Circuits - Periodic Functions, Average Rms ValuesDocument16 pagesAC Circuits - Periodic Functions, Average Rms ValuesRaaz Veer YadavNo ratings yet
- GROUP 2 - General Physics II Microwave RadiationDocument9 pagesGROUP 2 - General Physics II Microwave RadiationRheyven JuanNo ratings yet
- MD Product Catalogue PROXIMITY ENGDocument194 pagesMD Product Catalogue PROXIMITY ENGETIENNENo ratings yet